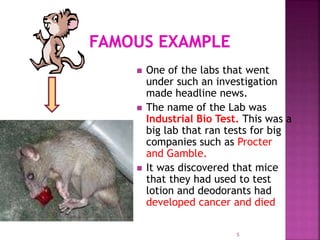
GLP is an FDA regulation that provides a framework for conducting laboratory studies in a planned, monitored, and archived manner to ensure data submitted is valid. GLP applies to nonclinical safety and efficacy studies of chemicals and pharmaceuticals to assure regulators that submitted data is accurate. In the 1970s, FDA investigations found many cases of poor laboratory practices, fraudulent activities, and inaccurate reports at toxicology labs, including one lab called Industrial Bio Test that falsified cancer data, leading to legal consequences. GLP was created in response to establish standards for test systems, record keeping, facilities, quality control, study performance and reporting.