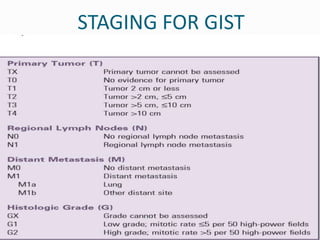
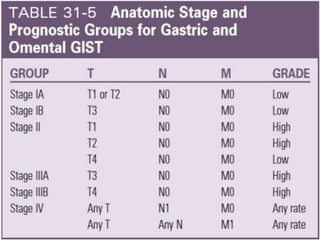
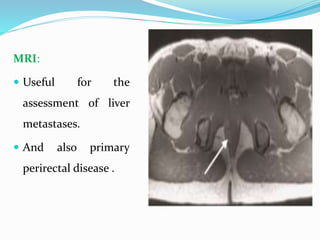
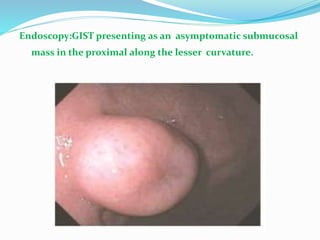
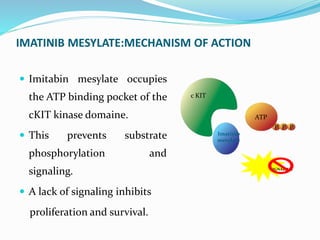
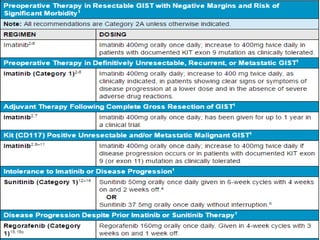
This document provides an overview of gastrointestinal stromal tumors (GISTs). It discusses the epidemiology, molecular mechanisms, clinical presentation, imaging, treatment, and outcomes of GISTs. GISTs are the most common mesenchymal tumors of the gastrointestinal tract. They originate from interstitial cells of Cajal and are characterized by gain-of-function mutations in KIT or PDGFRA genes. Treatment involves surgery when possible as well as targeted therapy with drugs like imatinib, which inhibits KIT and other oncogenic proteins. Close monitoring is important after treatment due to the risk of recurrence.