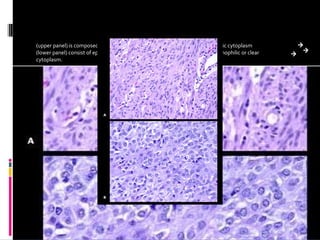
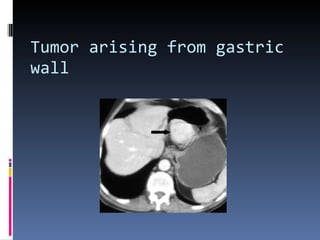
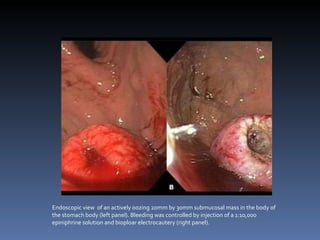
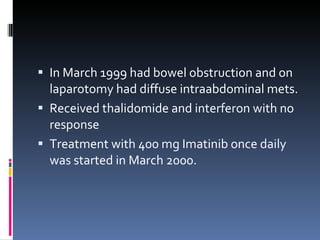
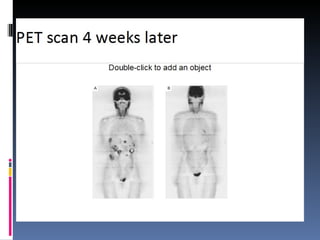
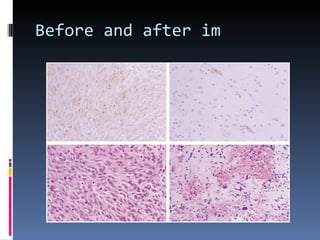
- A 62-year-old male presented with severe headache, chest pain, and nausea. Endoscopy revealed a 3cm submucosal gastric mass which was biopsy and found to be a gastrointestinal stromal tumor (GIST) positive for CD117 and CD34.
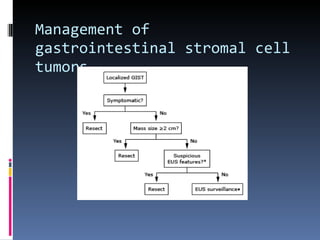
- GISTs are typically treated with the tyrosine kinase inhibitor imatinib, which has increased survival rates. For this patient, the mass was removed surgically and he was prescribed adjuvant imatinib therapy given the tumor size. Long term follow-up is needed after complete resection of GISTs.