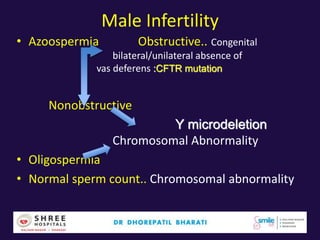
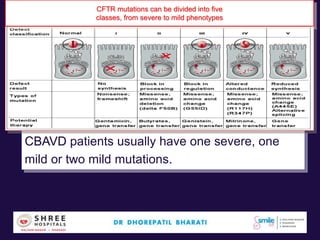
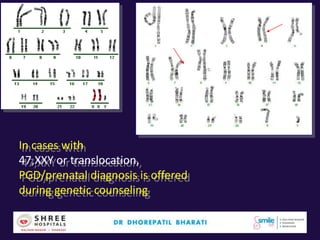
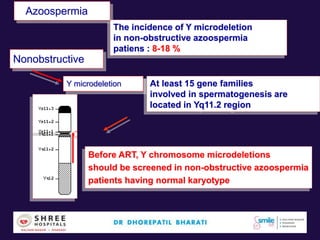
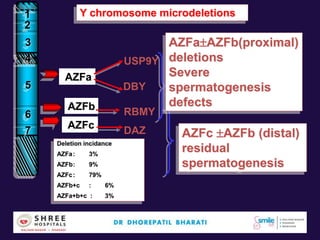
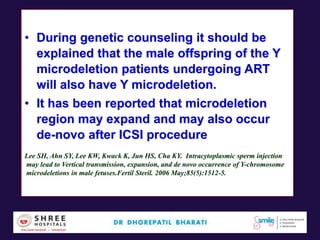
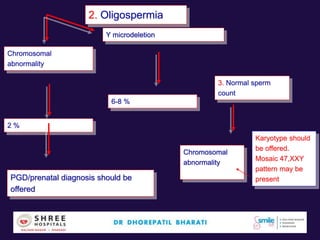
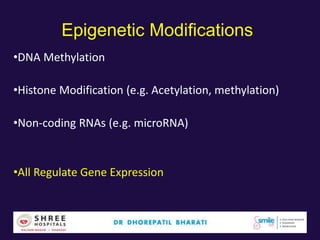
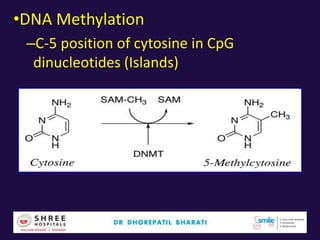
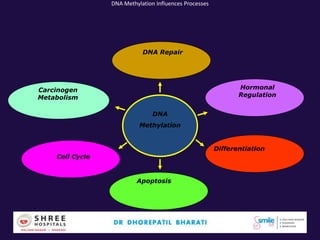
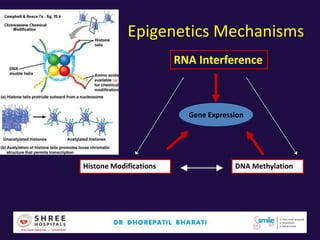
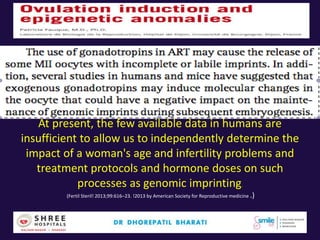
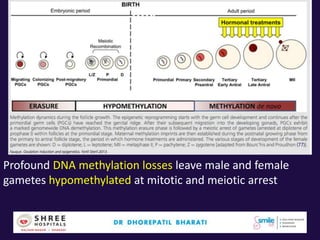
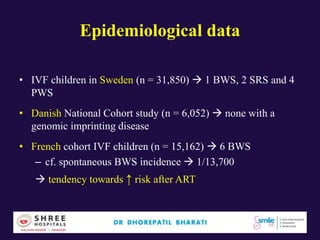
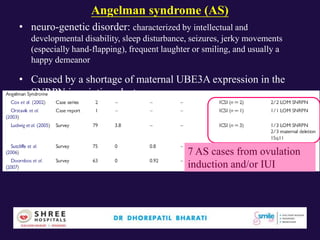
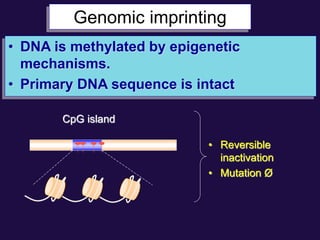
The document discusses the genetic and epigenetic factors impacting infertility, focusing on how these factors can be minimized through genetic counseling. It highlights the importance of understanding the genetic causes of male and female infertility, the risks of genetic disorders in children conceived through assisted reproductive technologies (ART), and the potential epigenetic alterations that may occur due to ART procedures. Key themes include the role of genomic imprinting, epigenetic mechanisms, and the implications for both immediate outcomes and long-term health in offspring.