Recommended
PPT
Accuracy-and-precision.ppt
PPTX
LESSON-2-Accuracy-and-Precision.pptxdsdssd
PPT
measurement-and-data-processing-errors-and-uncertainties.ppt
PPTX
tool 3 mathssolikeibmathsishardrightthisisresourcesforyou.pptx
PDF
Accuracy & uncertainty
PPTX
Errors and uncertainities net
PPTX
Uncertainty in laboratory experiments and random and systematic errors
PPTX
Errors and uncertainty analysis - Physics A level
PPTX
Measurement-Uncertainties ppt FOR GRADE 12 STUDENTS
PPTX
ACCURACY AND PRECISION general physics.pptx
PPTX
PDF
GeneralPhysics-L3-Experimental-Errors.pdf
PPT
Presentation on Measurement Uncertainity.ppt
PPT
Accuracy, Precision Measurement
PPTX
Errors and uncertainties in physics
PPTX
accuracy and precision in General Chemistry
PPT
accuracyprecisionerrordfgjgkhghv_notes.ppt
PPTX
1.2 - Uncertainties and errors.pptx
PPTX
PPTX
33a02983-ccce-4368-91db-71cec0f82820.pptx
PPTX
Edexcel IAL Unit 3 - experimental physics.pptx
PPTX
Precision and Accuracy General Physics I.pptx
PDF
manual.pdf and details about errors analysis
PPT
accuracyprecisionerror_notes physics.ppt
PPT
Calibration of Uncertainty Measurement .
PPTX
Errors in Chemistry ANALYTICAL CHEMISTRY (Errors in Chemical Analysis).pptx
PPTX
PPTX
Error Analysis and Measurements Chapter 1.pptx
PPT
PROTEINS POWERPOINT PRESENTATION. .ppt
PPTX
PTA-MEETING-DECEMBER-6 POWERPOINT PRESENTATION.pptx
More Related Content
PPT
Accuracy-and-precision.ppt
PPTX
LESSON-2-Accuracy-and-Precision.pptxdsdssd
PPT
measurement-and-data-processing-errors-and-uncertainties.ppt
PPTX
tool 3 mathssolikeibmathsishardrightthisisresourcesforyou.pptx
PDF
Accuracy & uncertainty
PPTX
Errors and uncertainities net
PPTX
Uncertainty in laboratory experiments and random and systematic errors
PPTX
Errors and uncertainty analysis - Physics A level
Similar to GENERAL CHEMISTRY ACCURACY AND PRECISION.pptx
PPTX
Measurement-Uncertainties ppt FOR GRADE 12 STUDENTS
PPTX
ACCURACY AND PRECISION general physics.pptx
PPTX
PDF
GeneralPhysics-L3-Experimental-Errors.pdf
PPT
Presentation on Measurement Uncertainity.ppt
PPT
Accuracy, Precision Measurement
PPTX
Errors and uncertainties in physics
PPTX
accuracy and precision in General Chemistry
PPT
accuracyprecisionerrordfgjgkhghv_notes.ppt
PPTX
1.2 - Uncertainties and errors.pptx
PPTX
PPTX
33a02983-ccce-4368-91db-71cec0f82820.pptx
PPTX
Edexcel IAL Unit 3 - experimental physics.pptx
PPTX
Precision and Accuracy General Physics I.pptx
PDF
manual.pdf and details about errors analysis
PPT
accuracyprecisionerror_notes physics.ppt
PPT
Calibration of Uncertainty Measurement .
PPTX
Errors in Chemistry ANALYTICAL CHEMISTRY (Errors in Chemical Analysis).pptx
PPTX
PPTX
Error Analysis and Measurements Chapter 1.pptx
More from LORENZORojiKentB
PPT
PROTEINS POWERPOINT PRESENTATION. .ppt
PPTX
PTA-MEETING-DECEMBER-6 POWERPOINT PRESENTATION.pptx
PPTX
Earth and Life Science-Pre-Test.....pptx
PPTX
GChem2_Pretest_CreativeDesign.......pptx
PPTX
GChem2_Pretest_WithChoices Grade 12 .pptx
PPTX
Chemical_Thermodynamics_Presentation.pptx
PPTX
Atoms-Orbitals PowerPoint presentation.pptx
PPTX
PLANT AND ANIMAL REPRODUCTION PRESENTATION.pptx
PPTX
9.REPRODUCTION IN PLANTS PRESENTATION.pptx
PPTX
10.REPRODUCTION IN ANIMALS PRESENTATION.pptx
PPT
PERIODIC TRENDS PowerPoint presentation.ppt
PPTX
TYPES AND EVIDENCES OF CHEMICAL REACTION.pptx
PPTX
HYDROMETEOROLOGICAL HAZARD.powerpoint.pptx
PPTX
REDOX REACTION and MASS RELATIONSHIP.pptx
PPTX
Cell-organelles powerpoint presentation.pptx
PPTX
PRESENTATION ON MOLE CONCEPT PART 1.pptx
PDF
CHEMISTRY-LABORATORY-MANUAL PRESENTATION.pdf
PPT
Chemistry 3.1 Notes (Accuracy, precision, error and sig figs).ppt
PPTX
ORIGIN OF THE UNIVERSE POWERPOINT 1.pptx
PPTX
GENERAL CHEMISTRY 1STATES-OF-MATTER.pptx
Recently uploaded
PPTX
West Hatch High School - GCSE Media Studies
PPT
West Hatch High School - GCSE History Option
PDF
Information about the author Shashi Deshpande
PDF
Information about Presentation strategies
PPTX
literary theory and criticism by Vivek p
PPTX
Master of Punjabi Short Story "kartar singh duggal
PDF
Beak Modifications by Dr. Ramzan Virani pptx.pdf
PPTX
Definition of communication skills and it's process.
PPTX
HABIT. Cognitive process. I Semester Shilpa Hotakar pptx
PPTX
Toba Tek Singh - Visualising Partition through Manto's Lens
PPTX
Drugs modulating serotonergic system.pptx
PPTX
West Hatch High School -- GCSE Geography
PDF
U.S. Departments of Education and Treasury fact sheet
PPTX
" Jaya : Silence as Resistance " - That long silence
PPTX
"Aristotle : Father Of Western Philosophy"
PPTX
That long silence - Novel by Shashi Deshapande
PPTX
28 January 2026 Rebecca Frankum Are high-stakes exams and assessments still r...
PPTX
YSPH VMOC Special Report - Measles - The Americas 1-25-2026
PPTX
Grade 9 and 10 learner Fuse and Switch.pptx
PPTX
Powerpoint for testing in embed test with Sway
GENERAL CHEMISTRY ACCURACY AND PRECISION.pptx 1. 2. WHY DO ACCURACY AND PRECISION MATTER?
●Important in Science, Engineering and
everyday life.
●Helps improve reliability and quality of
measurements.
●Used in experiments, data collection,
and quality
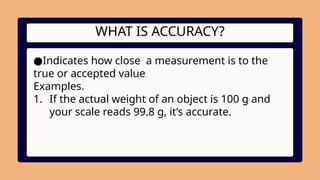
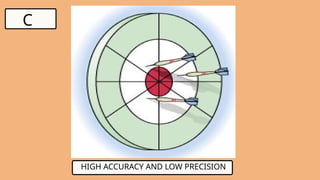
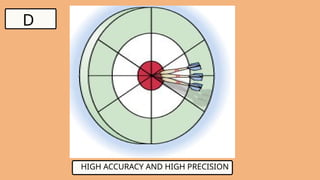
3. WHAT IS ACCURACY?
●Indicates how close a measurement is to the
true or accepted value
Examples.
1. If the actual weight of an object is 100 g and
your scale reads 99.8 g, it’s accurate.
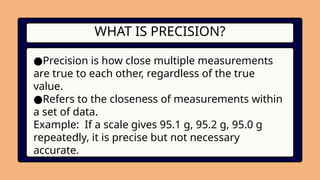
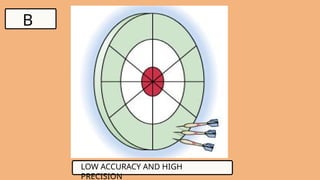
4. WHAT IS PRECISION?
●Precision is how close multiple measurements
are true to each other, regardless of the true
value.
●Refers to the closeness of measurements within
a set of data.
Example: If a scale gives 95.1 g, 95.2 g, 95.0 g
repeatedly, it is precise but not necessary
accurate.
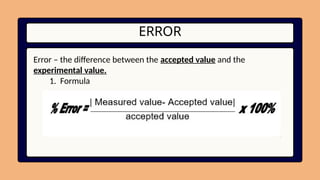
5. 6. 7. 8. 9. 10. ERROR
Error – the difference between the accepted value and the
experimental value.
1. Formula
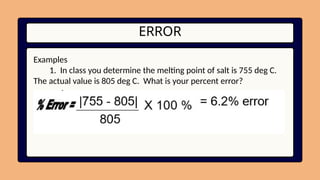
11. ERROR
Examples
1. In class you determine the melting point of salt is 755 deg C.
The actual value is 805 deg C. What is your percent error?
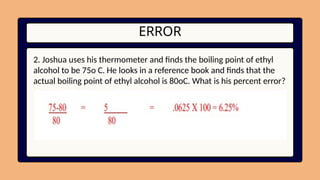
12. ERROR
2. Joshua uses his thermometer and finds the boiling point of ethyl
alcohol to be 75o C. He looks in a reference book and finds that the
actual boiling point of ethyl alcohol is 80oC. What is his percent error?