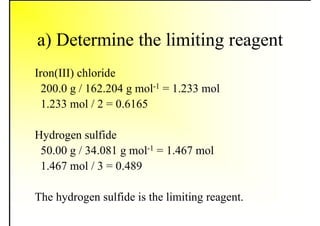
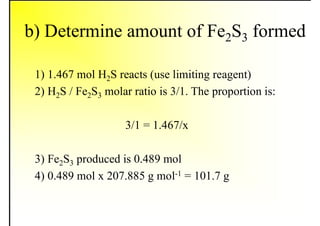
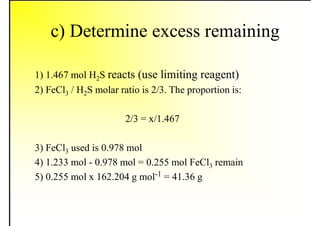
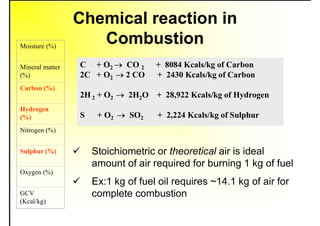
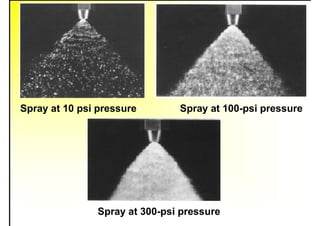
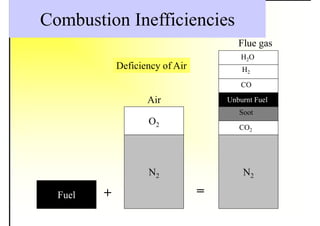
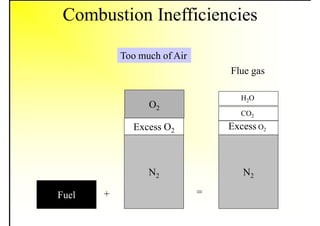
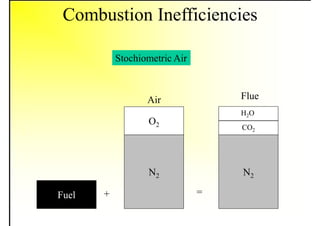
1. The document discusses fuels and combustion, including different types of fuels, their properties, storage, handling, and combustion principles.
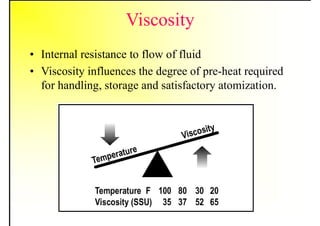
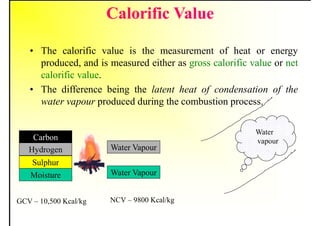
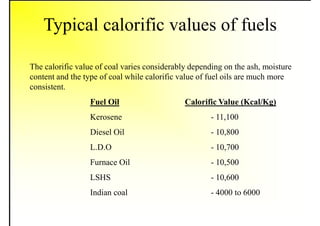
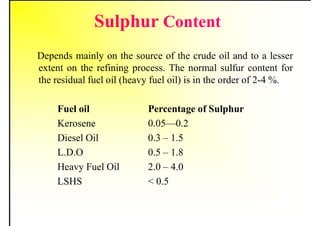
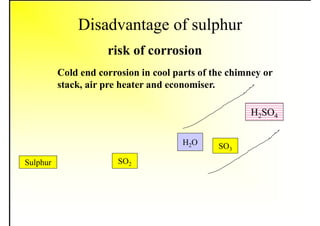
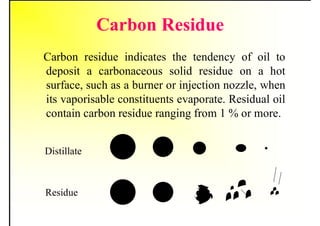
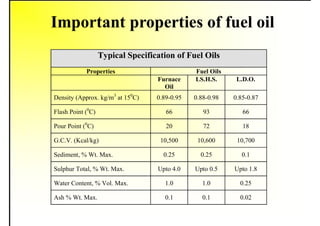
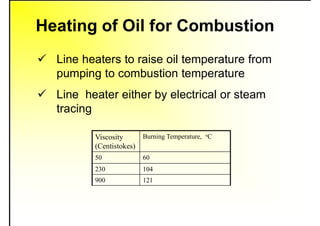
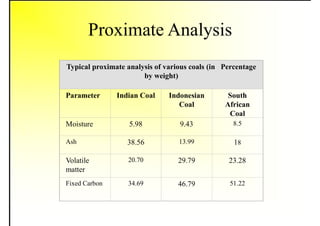
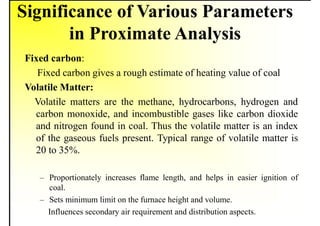
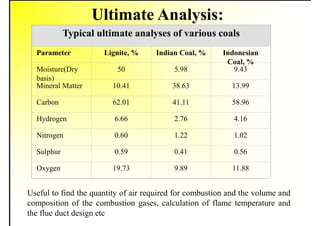
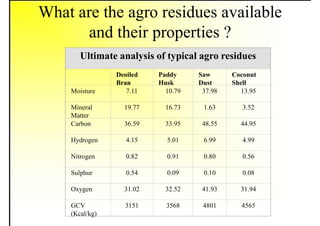
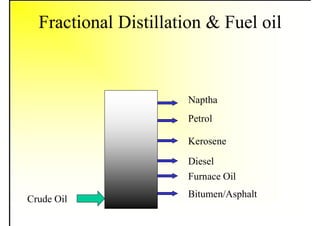
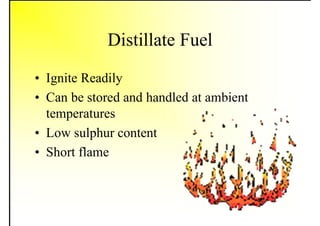
2. It describes the properties of various liquid fuels like density, viscosity, calorific value, and their impacts. It also discusses solid fuels like coal and their classification, proximate and ultimate analysis.
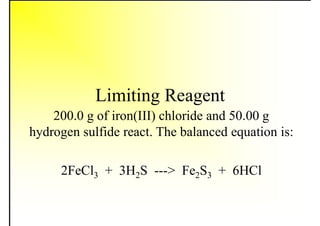
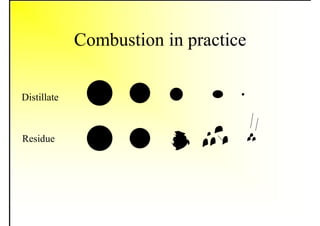
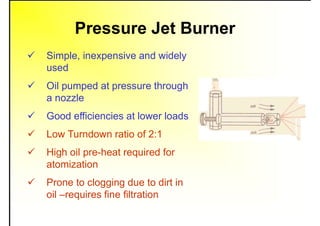
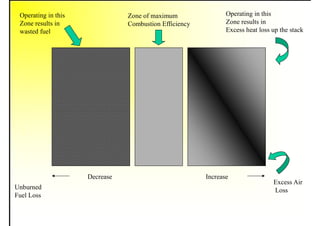
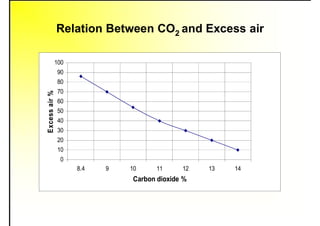
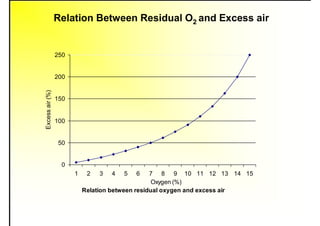
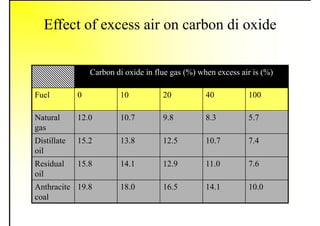
3. The key topics covered include fuel selection criteria, properties of important fuels like furnace oil and coal, storage and handling guidelines, and an introduction to combustion reactions.