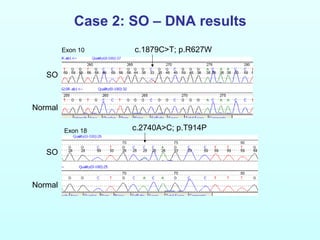
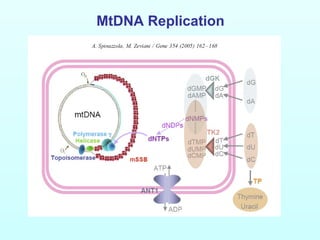
This document discusses autosomal disorders of mitochondrial DNA maintenance. It notes that these disorders involve defects in both the nuclear and mitochondrial genomes that can lead to tissue-specific oxidative phosphorylation defects and disease symptoms. Key points include:
1) Mutations in the POLG gene are a major cause of these disorders and can result in a broad spectrum of conditions from mild PEO to Alpers syndrome. Most POLG mutations are autosomal recessive.
2) Mutations in the PEO1 gene are a major cause of autosomal dominant PEO.
3) Mutations in the ANT1 gene are a relatively rare cause of autosomal dominant PEO.
Two case studies are described to illustrate POL

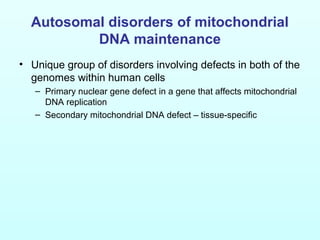
![Disorders associated with multiple
mtDNA deletions:
• Progressive external ophthalmoplegia with mitochondrial DNA
deletions
– Autosomal dominant
• PEOA1 – POLG (2001)
• PEOA2 – ANT1 (2000)
• PEOA3 – Twinkle (PEO1) (2001)
• PEOA4 – POLG2 (2006)
– Autosomal recessive
• PEOB1 – POLG (2001)
[note: POLG can cause AD or AR disease; any given mutation is
either associated with AD or AR disease]
• Other:
– MIRAS – POLG (2005)
– SANDO – POLG (2003)
– MNGIE – ECGF1 (thymidine phosphorylase) (1999)
– MNGIE without leukoencephalopathy – POLG (2003)
– Optic Atrophy ‘plus’ – OPA1 (2007)](https://image.slidesharecdn.com/frit13carlfratter3-150110123818-conversion-gate01/85/Fri-t13-carl_fratter_3-15-4-320.jpg)
![Disorders associated with
mtDNA depletion:
• Alpers syndrome
– POLG (2004)
• Hepatocerebral form
– DGUOK (2002)
– MPV17 (2006)
– PEO1 (2007)
• Encephalomyopathic form
– SUCLA2 (2005)
– RRM2B (2007)
• Myopathic form
– TK2 (2001)
[All autosomal recessive]](https://image.slidesharecdn.com/frit13carlfratter3-150110123818-conversion-gate01/85/Fri-t13-carl_fratter_3-15-5-320.jpg)

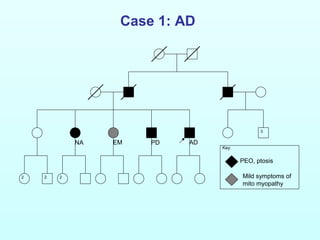
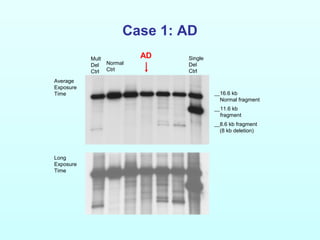
![Case 1: AD
NA PDEM AD
[R227W]+
[T251I;P587L]
[T251I;P587L]+
[T251I;P587L]
Inferred
[T251I;P587L] het
Inferred
[R227W]+[T251I;P587L]
[R227W]+
[T251I;P587L]
[R227W]+
[T251I;P587L]](https://image.slidesharecdn.com/frit13carlfratter3-150110123818-conversion-gate01/85/Fri-t13-carl_fratter_3-15-13-320.jpg)