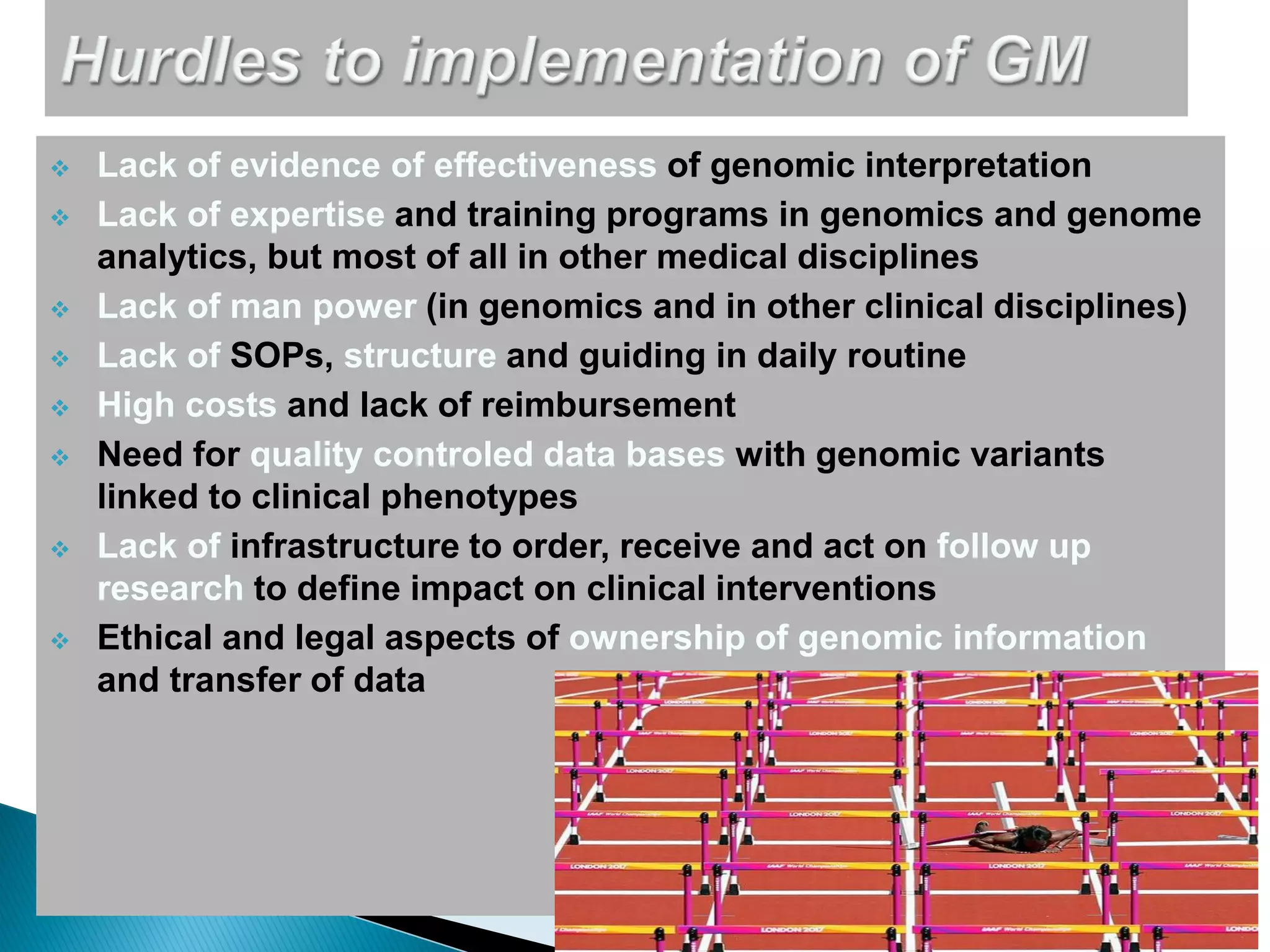
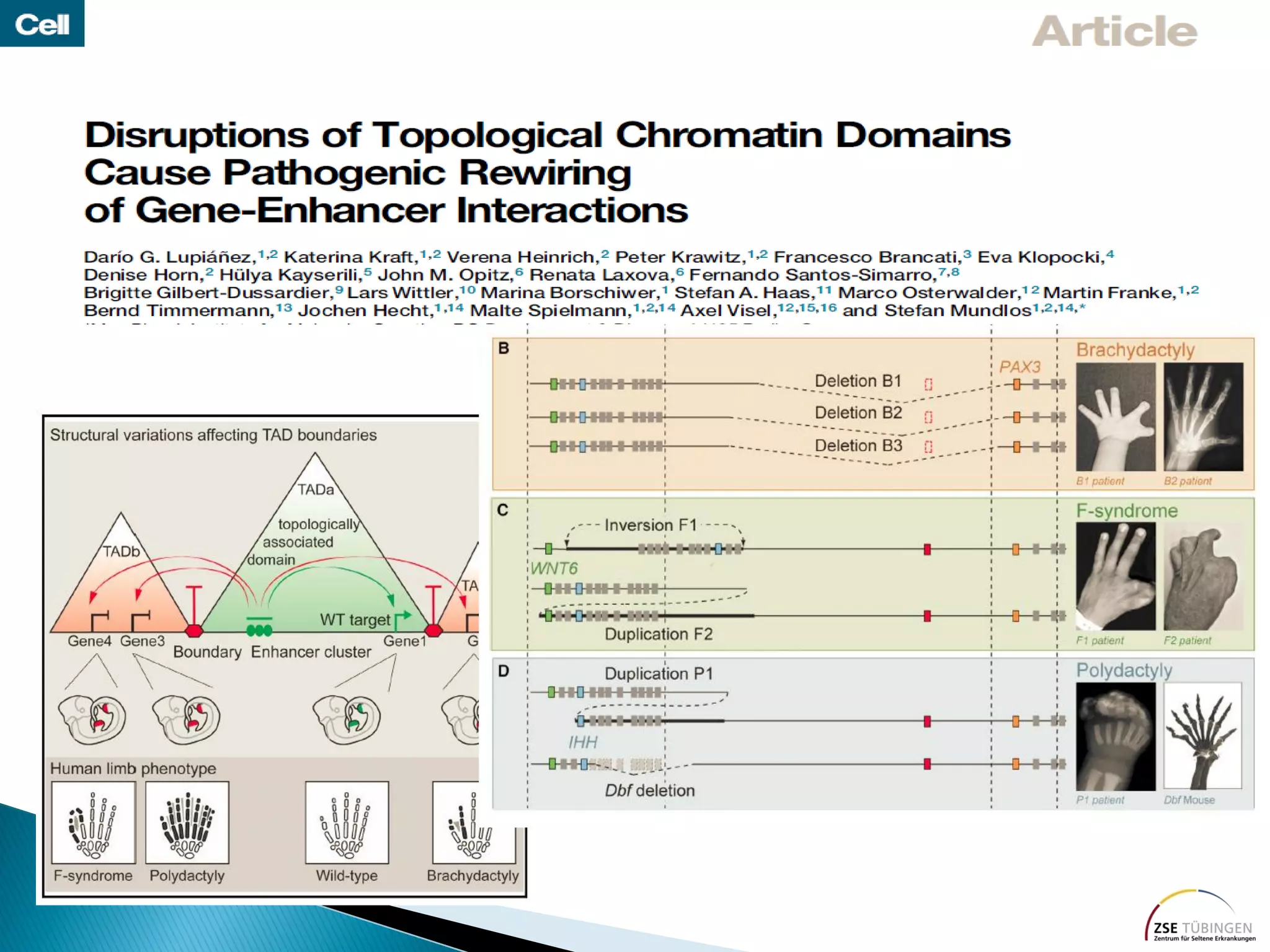
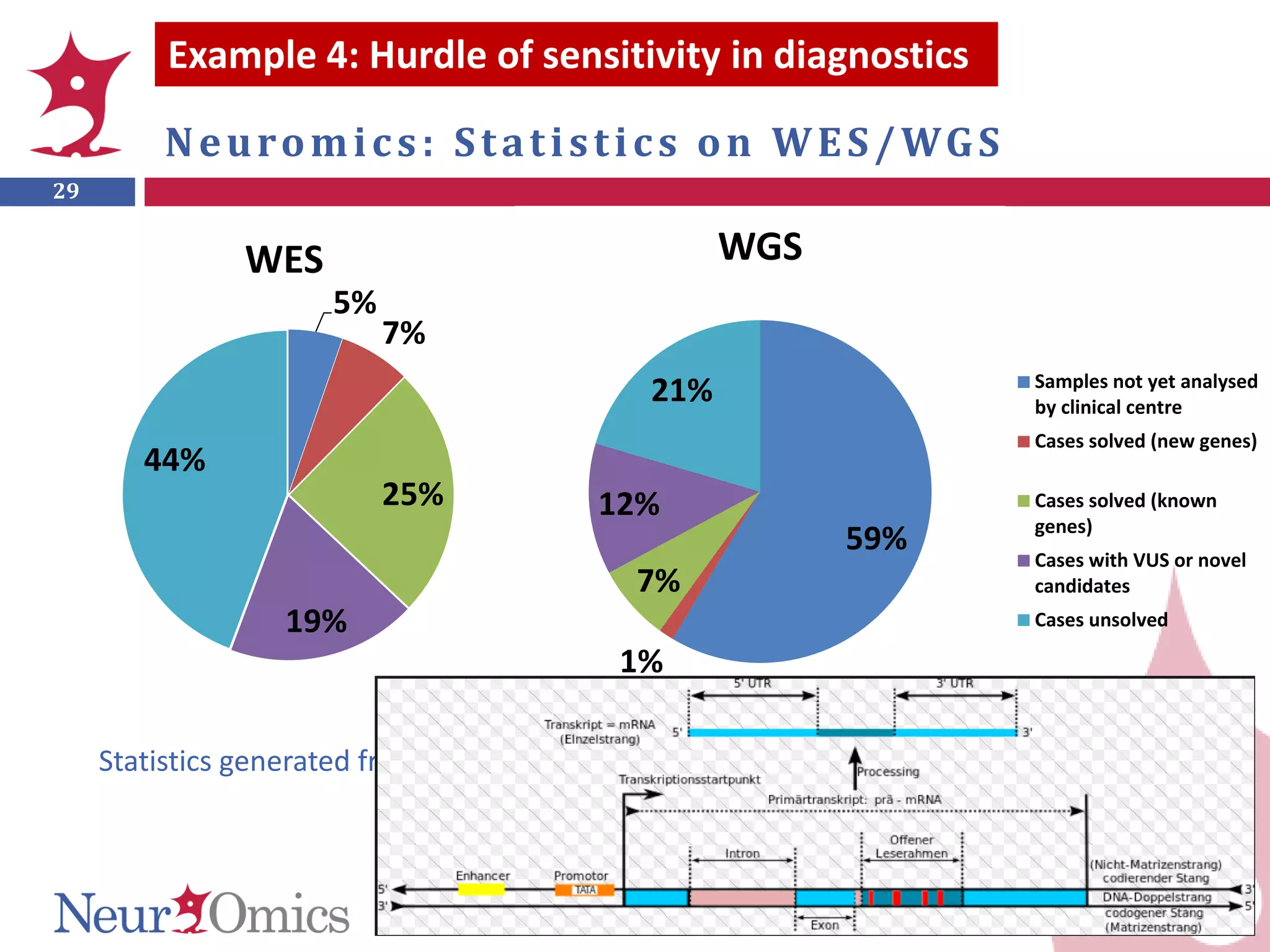
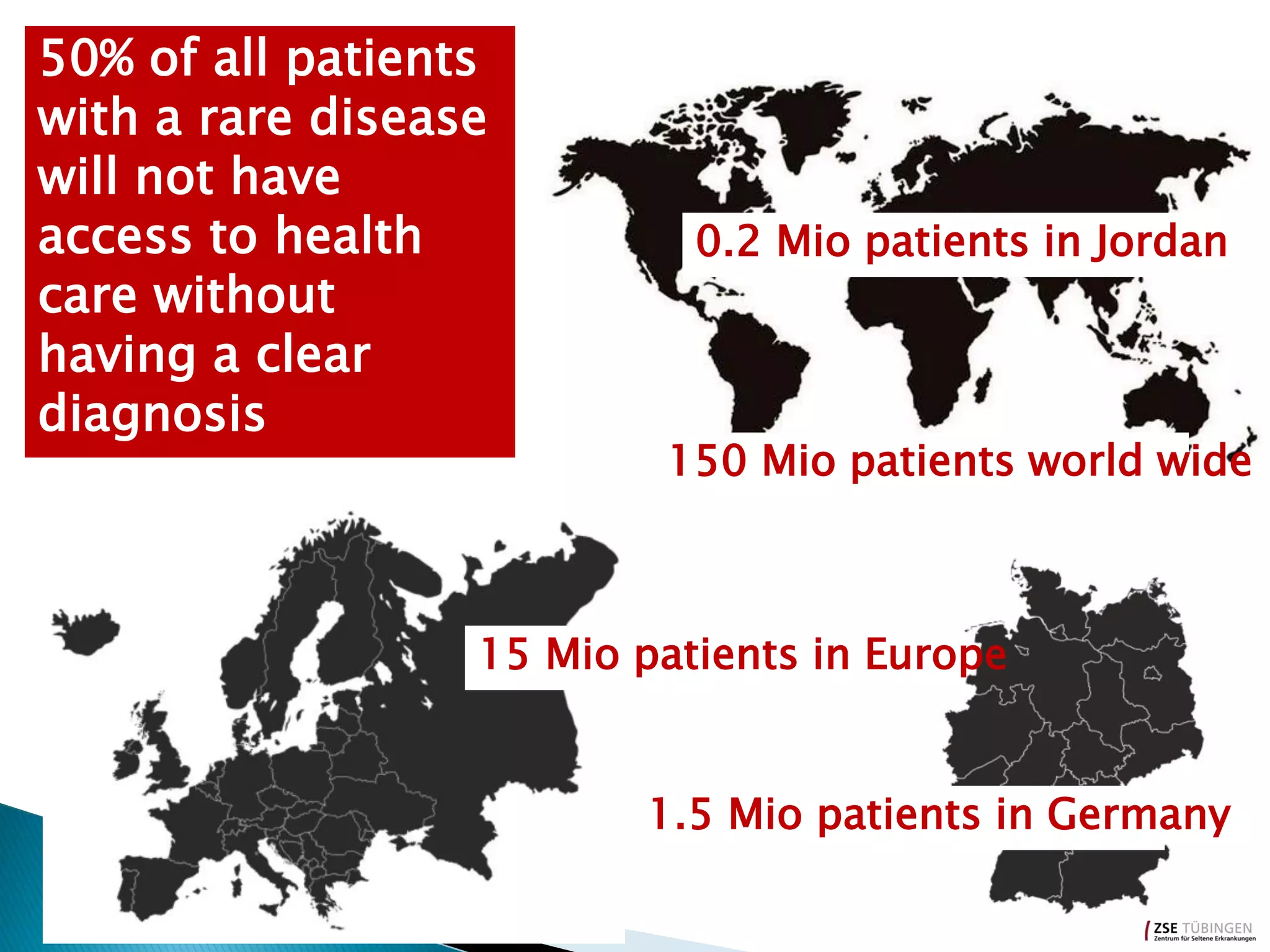
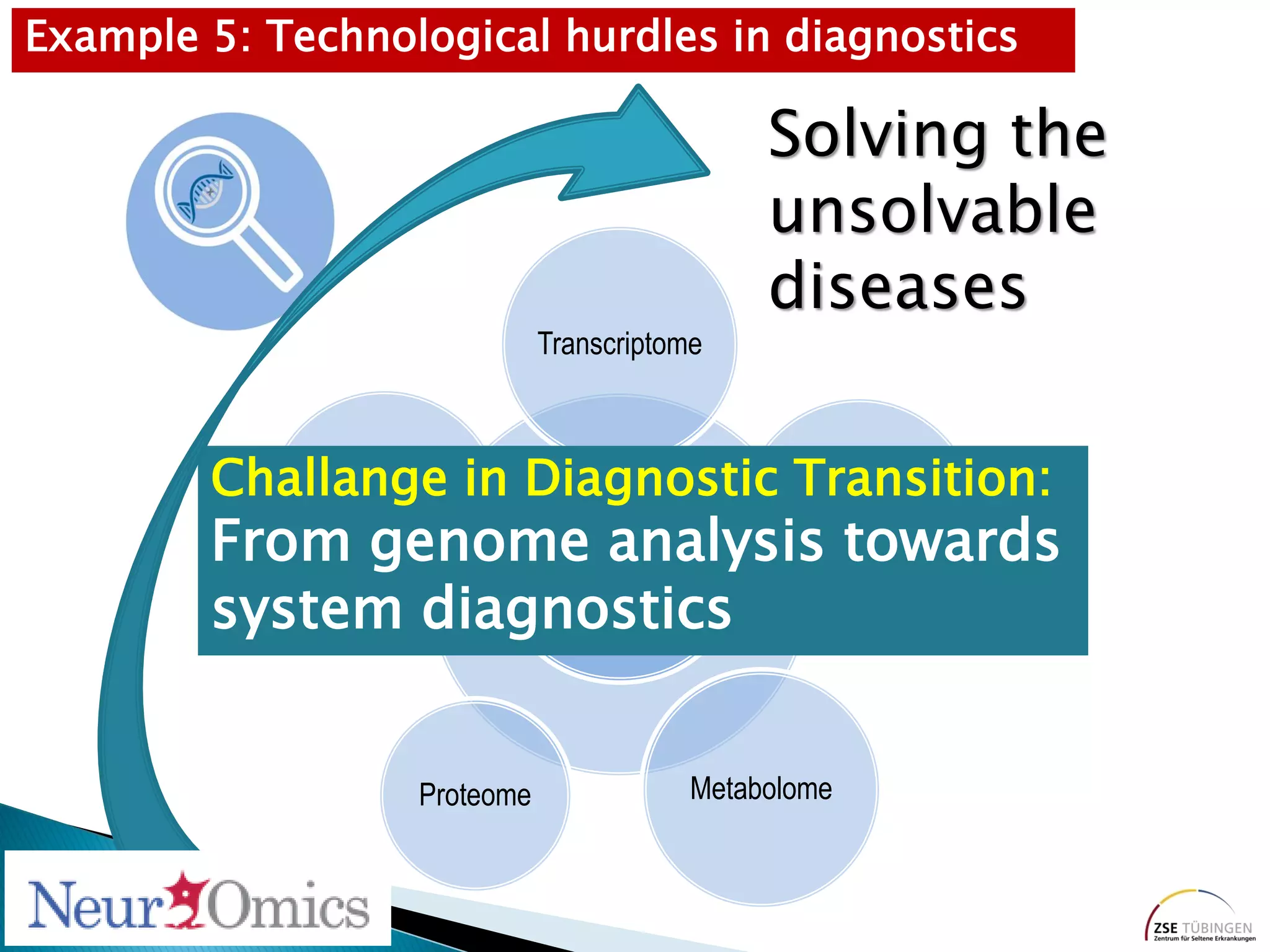
Genomic sequencing is expected to have the most impact in stratifying cancer patients for treatment, diagnosing genetic diseases to enable prevention, and providing information to reduce adverse drug reactions. However, diagnosing rare diseases using genomics faces many challenges, including a lack of expertise, infrastructure, and reimbursement for interpretation. Additionally, databases of genetic variants are often inaccurate, with around 27% of entries being wrong in their clinical implications. This leads to difficulties in diagnosis and determining treatment relevance. New interdisciplinary diagnostic concepts and centers are needed to help address these hurdles.





![Rare Neurometabolic Disorders
• A four-year-old girl, previously in a minimally conscious
state, began to communicate and walk with assistance
after nine weeks of treatment
• A three-year old girl showed developmental progress
• Immediate cessation of seizures in both
1
10
100
30 36 42 48 54 60 66
Seizure-free since
5 months
Age [month]
Tobias Haack](https://image.slidesharecdn.com/olaf-riess-state-of-play-in-diagnosis-180327145728/75/The-State-of-Play-in-Diagnosis-7-2048.jpg)