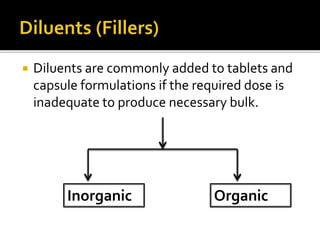
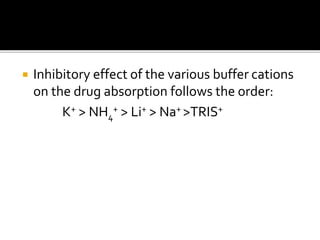
This document discusses how excipients and other formulation factors can influence drug absorption. It explains that excipients are added to ensure stability, uniformity, and optimal bioavailability. However, excipients can also impact absorption based on their type and amount. Factors like vehicle, diluents, binders, disintegrants and others are described in terms of how they can increase or decrease the rate of drug absorption from different dosage forms. The document emphasizes that proper selection of excipients and dosage form is important to maximize a drug's bioavailability.