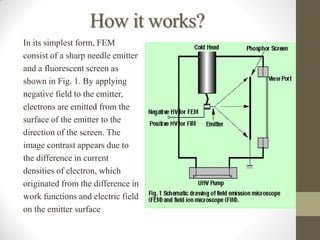
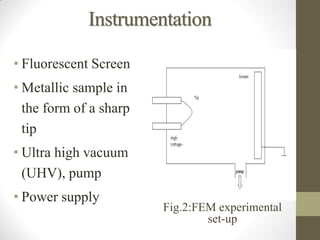
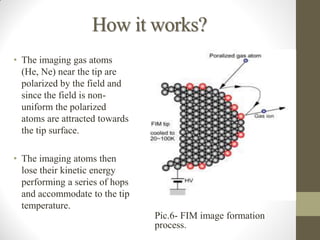
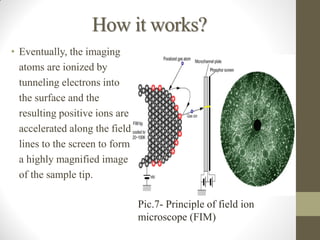
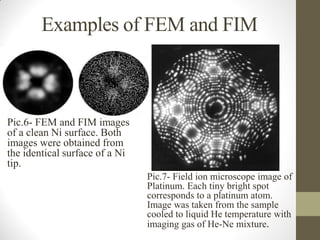
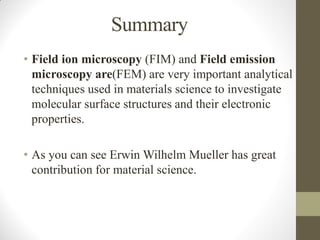
Field emission microscopy (FEM) and field ion microscopy (FIM) are essential analytical techniques in materials science used to analyze molecular surface structures and electronic properties. Both methods developed significant insights through the pioneering work of Erwin Wilhelm Mueller, with FEM providing near-atomic resolution and FIM enabling the observation of individual atoms. The document details their history, instrumentation, working conditions, and applications, highlighting their important roles in studying surface dynamics and characteristics.