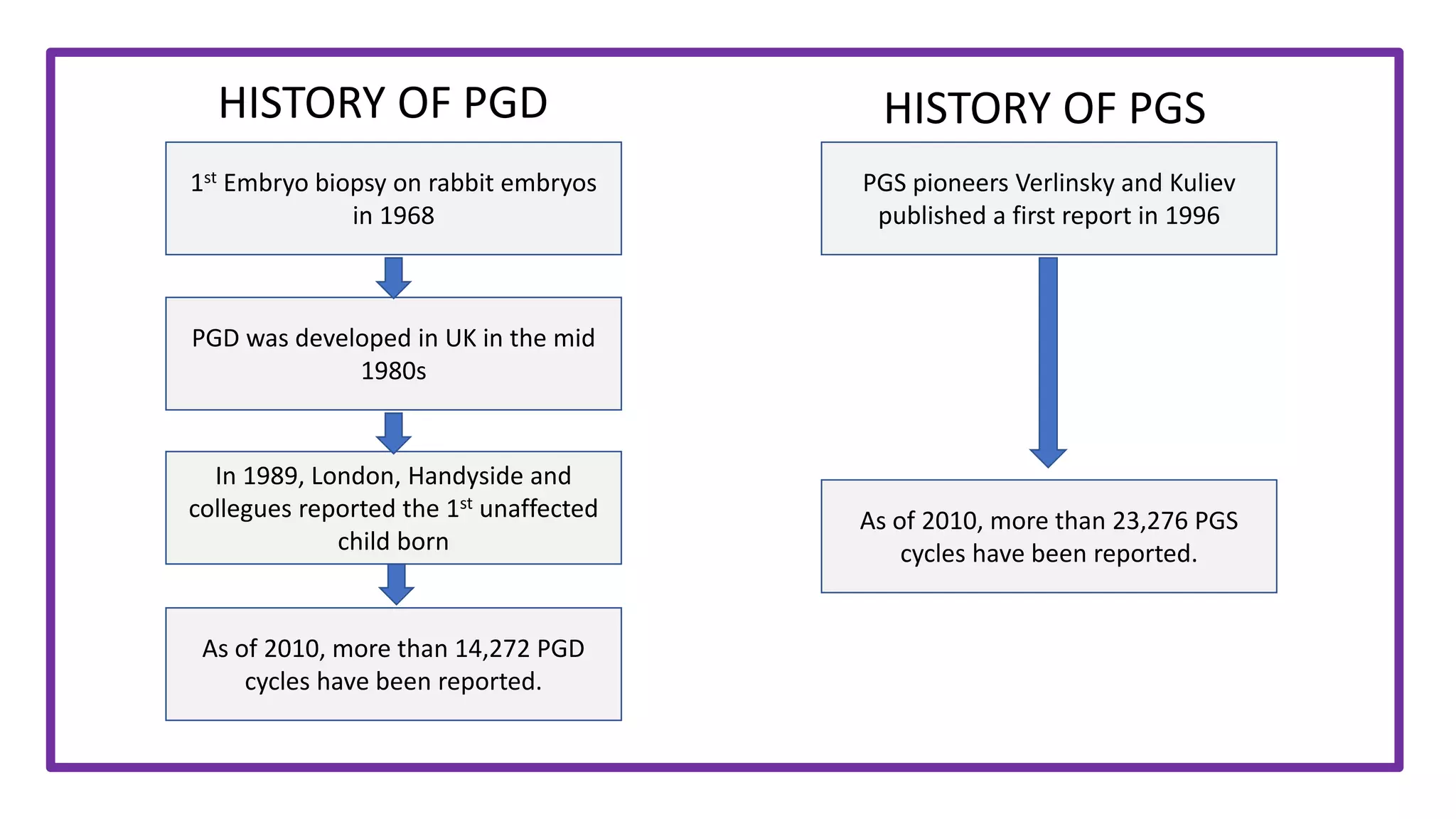
Dr. Laxmi Shrikhande is a leading fertility expert in India. She has held numerous leadership positions in national obstetrics and gynecology societies. Some of her accomplishments include receiving awards for women's health, publishing over 30 papers nationally and internationally, and conducting health programs that have reached over 25,000 women and girls. She is the Medical Director of Shrikhande Fertility Clinic in Nagpur, India.