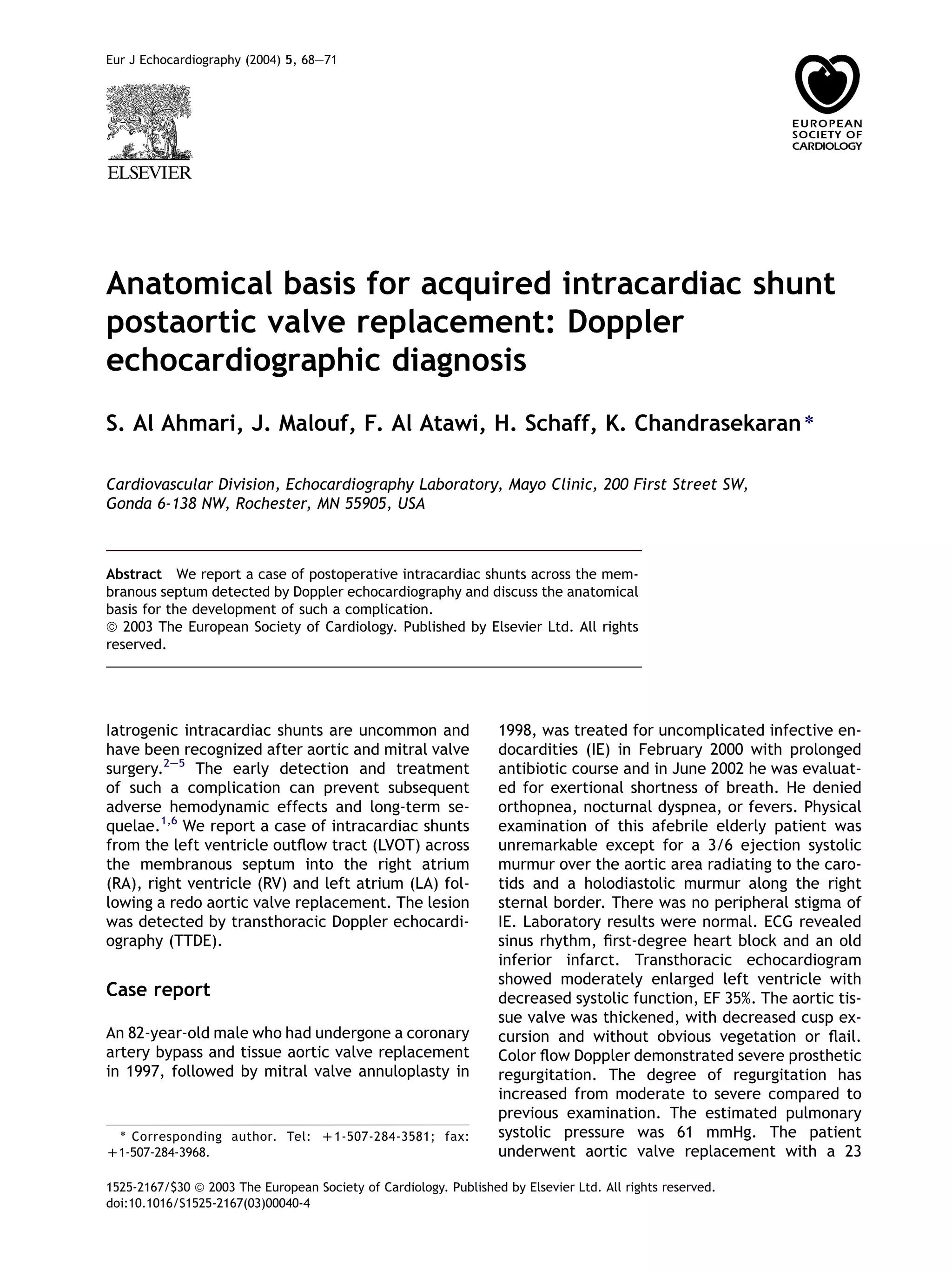
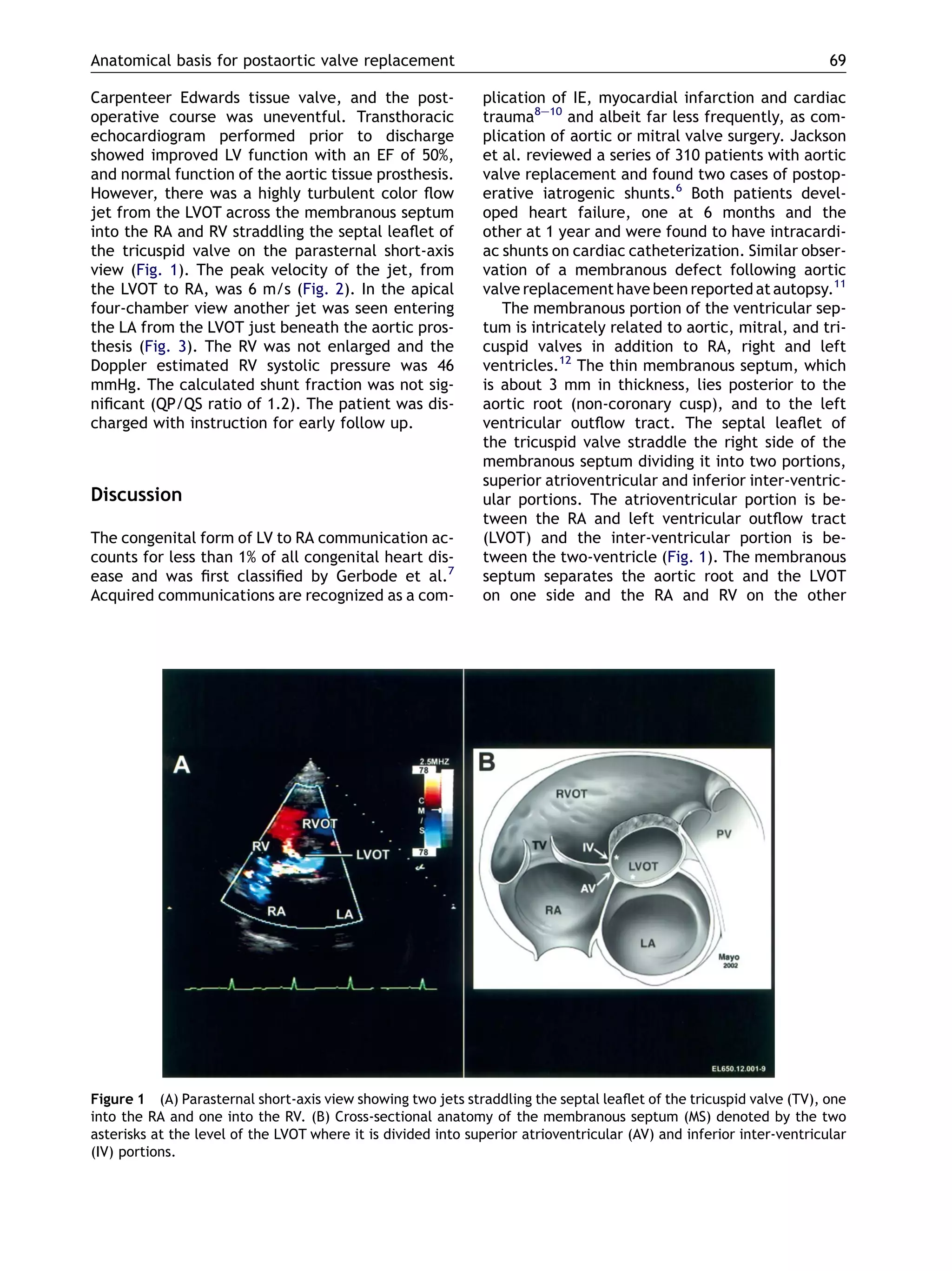
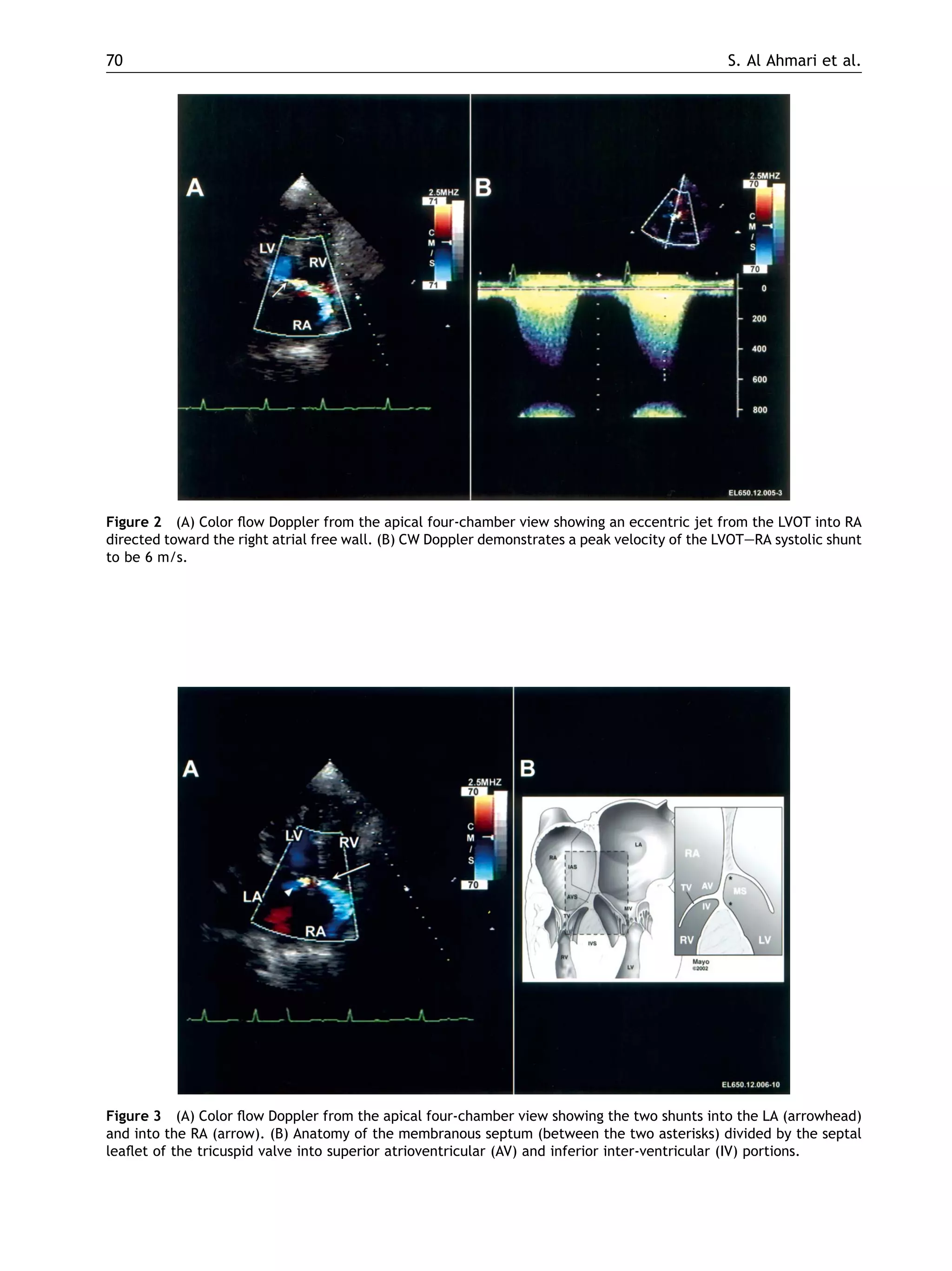
This case report describes an 82-year-old male who developed intracardiac shunts following a redo aortic valve replacement surgery. Doppler echocardiography detected shunts from the left ventricle outflow tract across the membranous septum into the right atrium, right ventricle, and left atrium. The anatomical basis for this complication is the proximity and relationship of the thin membranous septum to the aortic root, tricuspid valve, and ventricular chambers. Aggressive debridement during valve surgery can cause injury and necrosis of the membranous septum, leading to fistula formation over time. While the shunts were initially small and asymptomatic, they could enlarge