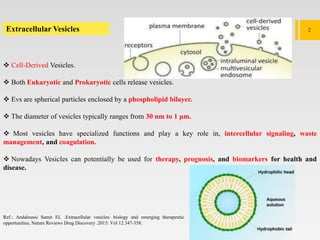
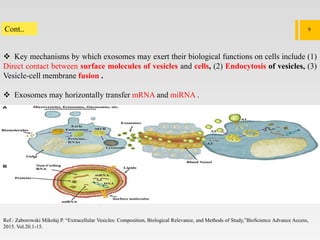
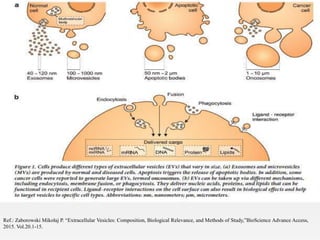
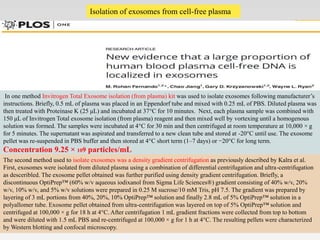
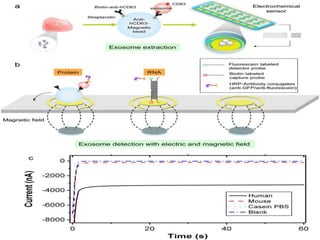
1. Extracellular vesicles (EVs) such as exosomes and microvesicles are naturally released from cells and have specialized functions including intercellular communication.
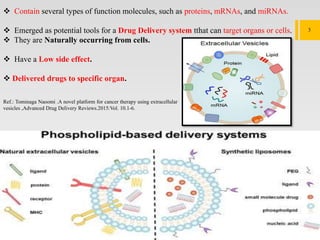
2. EVs contain proteins, mRNAs, miRNAs and other molecules. They have potential applications as drug delivery vehicles due to their ability to target specific cells and tissues with low toxicity.
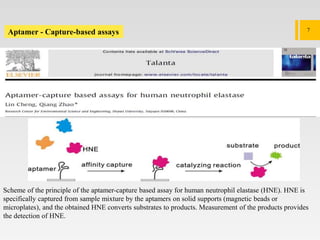
3. The document describes different types of EVs, their composition, biogenesis, isolation methods, and potential use for cancer therapy through targeted drug delivery via surface proteins on EVs.