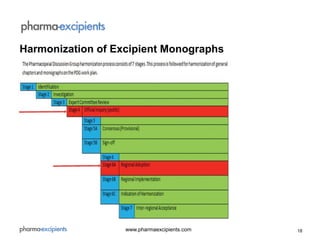
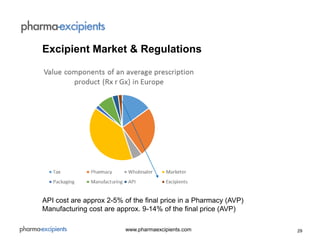
The document discusses pharmaceutical excipients, defined as substances other than active pharmaceutical ingredients that are critically evaluated for safety and included in drug delivery systems. It details the regulatory landscape, including guidelines from major pharmacopoeias and a focus on harmonizing standards to improve efficiency and safety in excipient production. The global excipients market is projected to grow significantly due to increasing pharmaceutical demands, although it faces challenges related to safety, quality, and regulatory compliance.