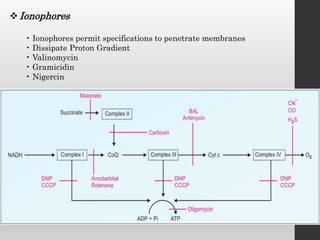
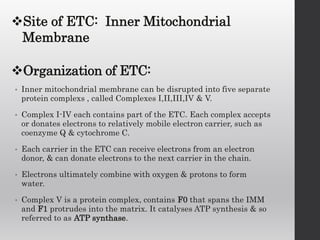
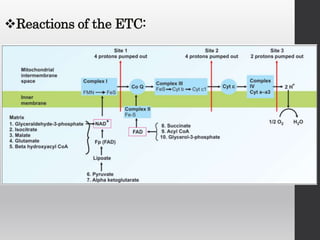
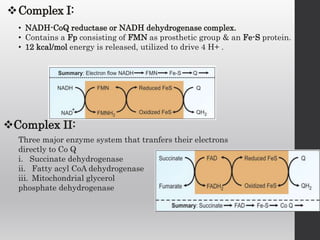
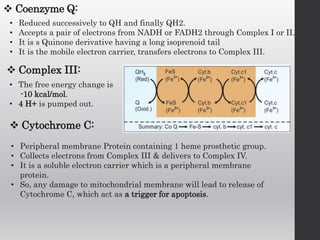
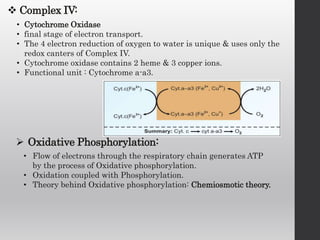
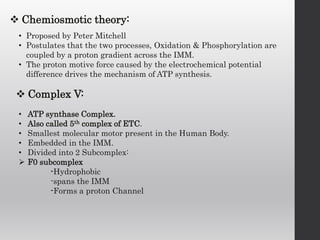
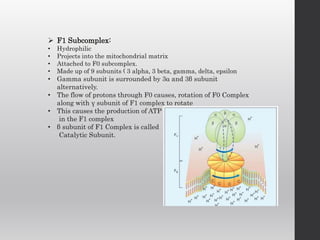
The document summarizes the structure and function of the electron transport chain (ETC) located in the inner mitochondrial membrane. It describes the five protein complexes (I-IV and V) that make up the ETC and how they facilitate the transfer of electrons from donors like NADH to final acceptors like oxygen. This electron transfer is coupled to the pumping of hydrogen ions across the membrane, creating a proton gradient that Complex V uses to synthesize ATP via oxidative phosphorylation. Various inhibitors are also outlined that can disrupt electron transfer at different complexes or uncouple the proton gradient from ATP production.
![ INHIBITORS OF ETC:
• Divided into
• Inhibitors of Electron transfer
• Inhibitors of Oxidative Phosphorylation
• Uncouplers of Oxidative Phosphorylation
• Ionophores
Inhibitors of Electron Transfer
Between NADH and CoQ [At Complex I]
• An insecticide and a fish Poison Rotenone
• Amobarbital which is a barbiturate
• Piericidin A.
Inhibitor of Complex II
• TTFA (Tri enoyl TriFluoroAcetone) a Fe2+ Chelating agent
• Carboxin
• Malonate, a competitive inhibitor of Succinate Dehydrogenase.
Between Cyt b and Cyt c [At Complex III]
• Antimycin A
• British Antilevisite [Dimercaprol]
Inhibitor at Cytochrome c Oxidase [Complex IV]
• CO
• Cyanide
• H2 S
• Sodium Azide](https://image.slidesharecdn.com/etc-240330105441-e89f2277/85/ETC-pptx-electron-transport-chain-PDUGMC-8-320.jpg)
![Inhibitors of Oxidative Phosphorylation
Atractyloside
By inhibiting the transporter of ADP into and ATP out of the
mitochondrion.
Oligomycin an Antibiotic
Completely blocks oxidation and phosphorylation. By blocking the
flow of protons through F0 Complex of ATP Synthase
Uncoupler of Oxidative Phosphorylation
Mechanism of Action—Disruption of Proton Gradient across the
inner mitochondrial membrane
• 2,4 Dinitrophenol
• Dinitrocresol
• FCCP [Fluoro Carbonyl Cyanide Phenyl hydrazine]
• Aspirin in high dose
Physiological Uncouplers : Thyroxine (in high doses),
Thermogenin.](https://image.slidesharecdn.com/etc-240330105441-e89f2277/85/ETC-pptx-electron-transport-chain-PDUGMC-9-320.jpg)