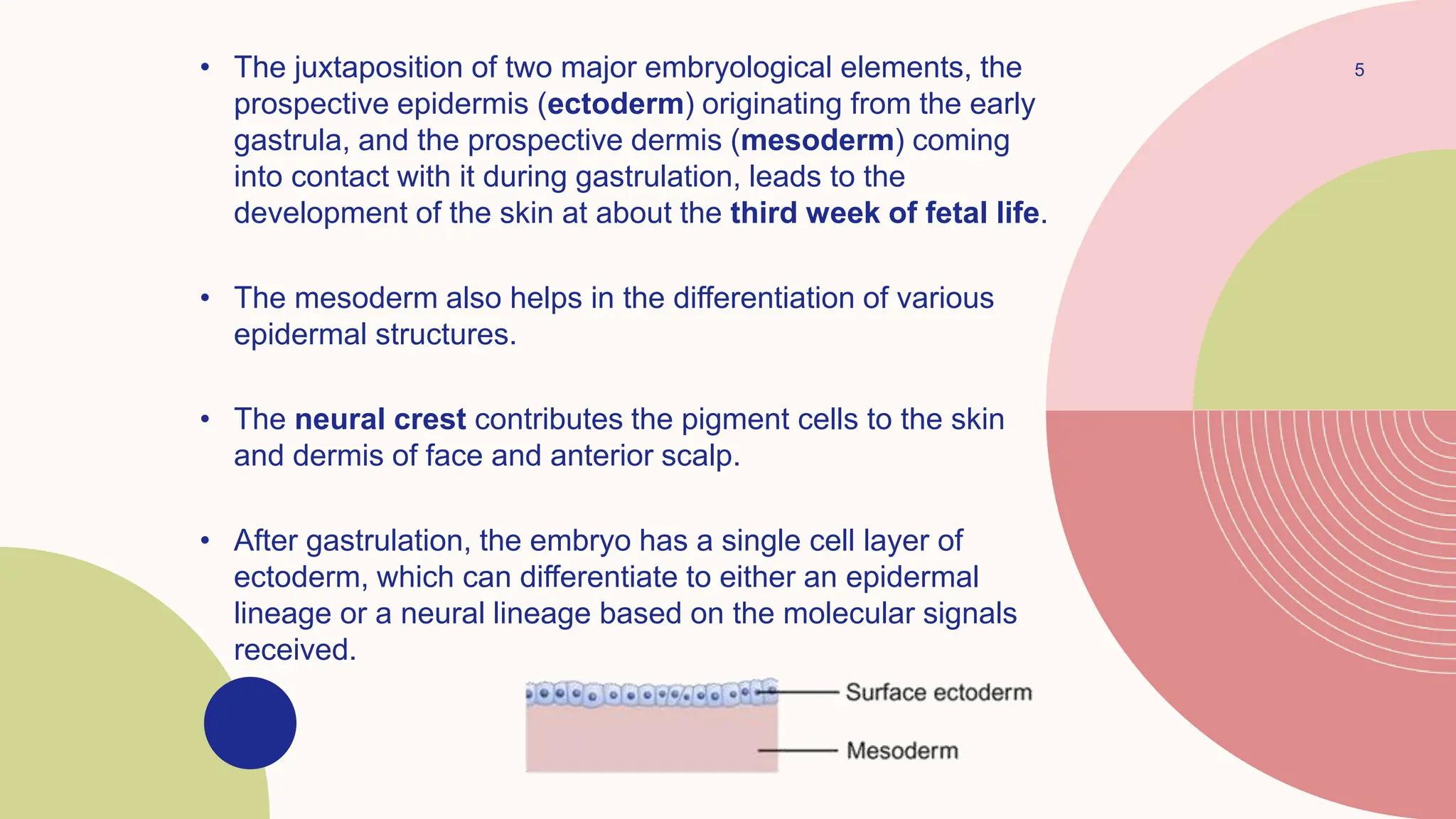
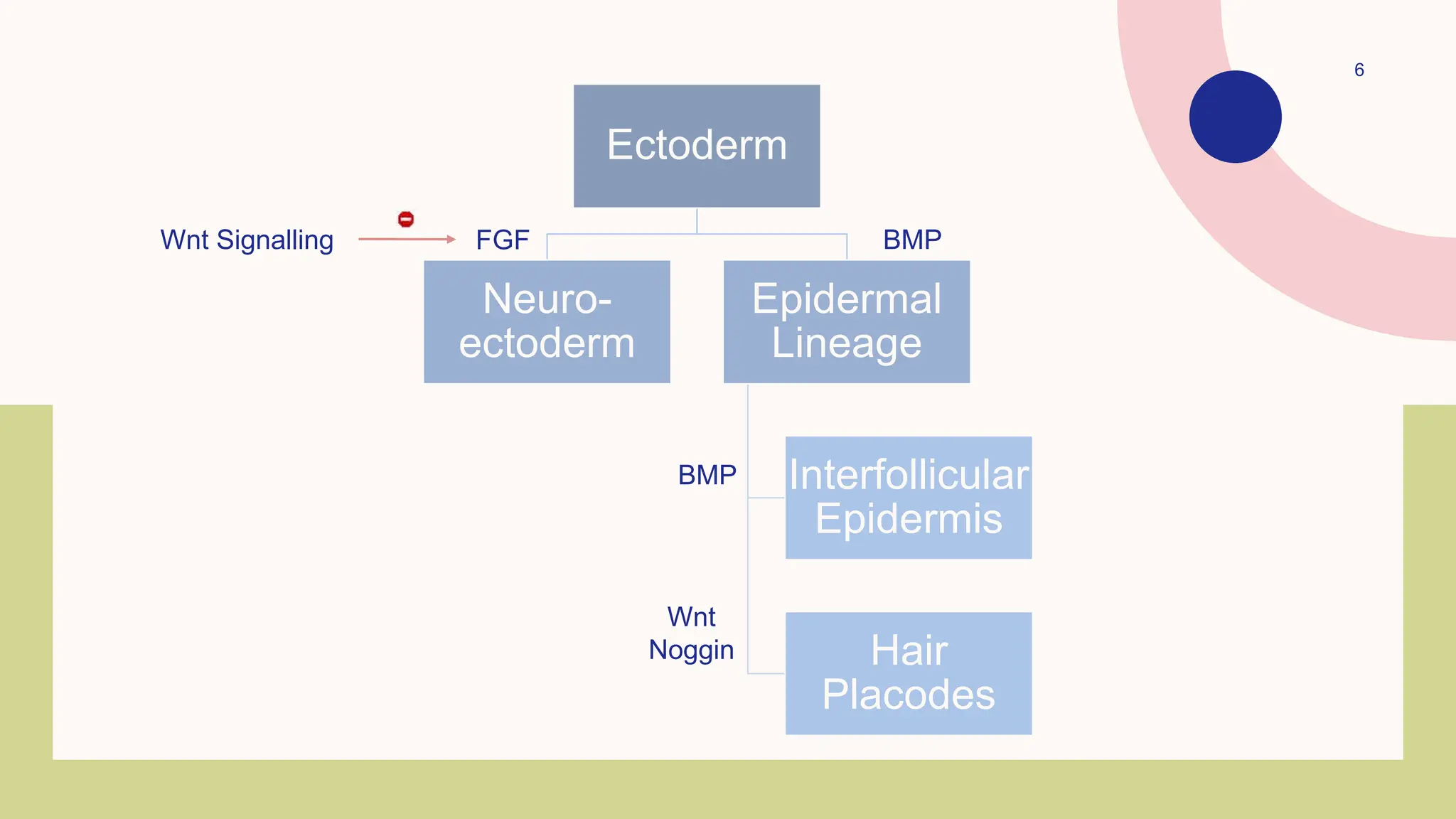
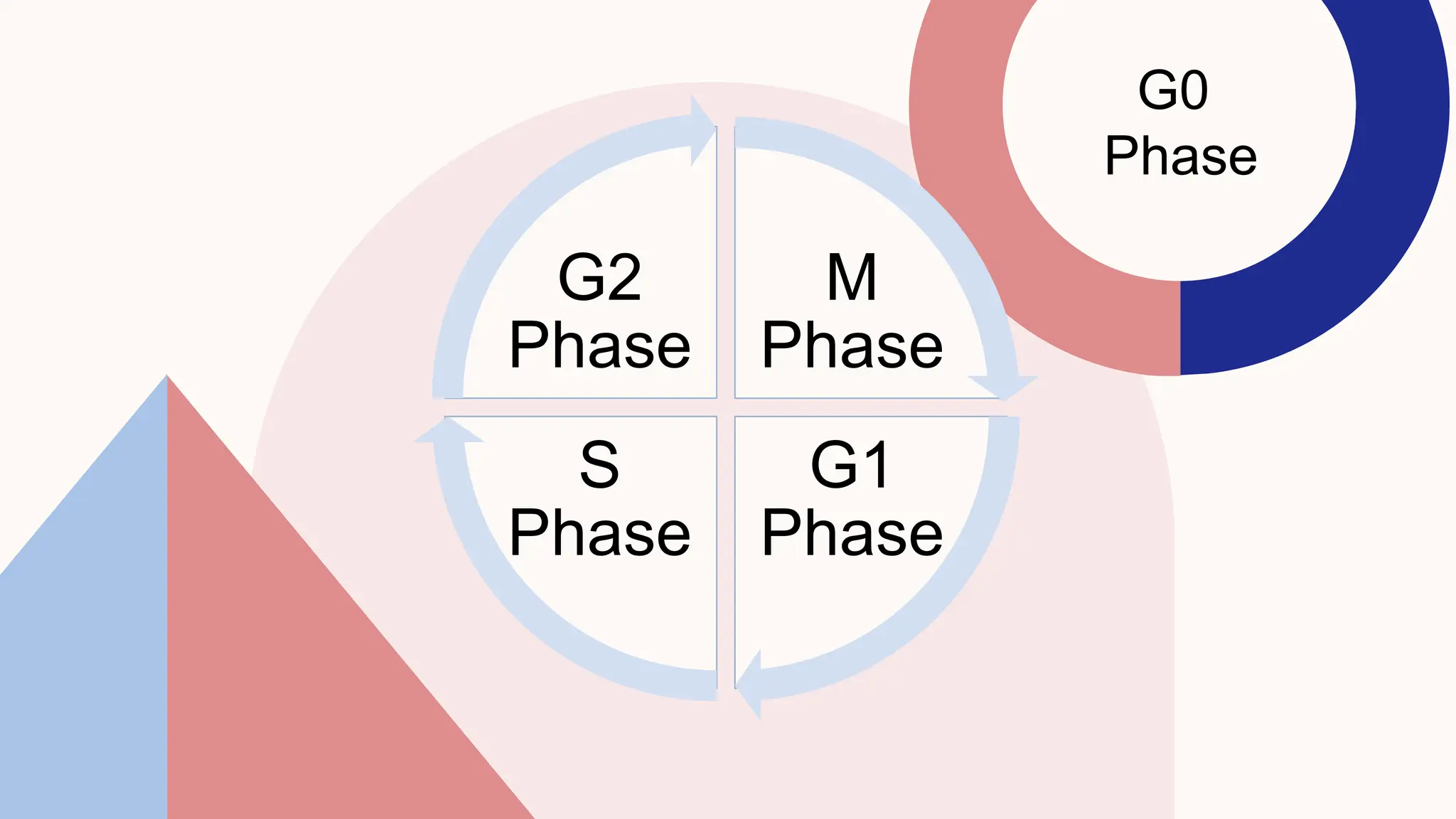
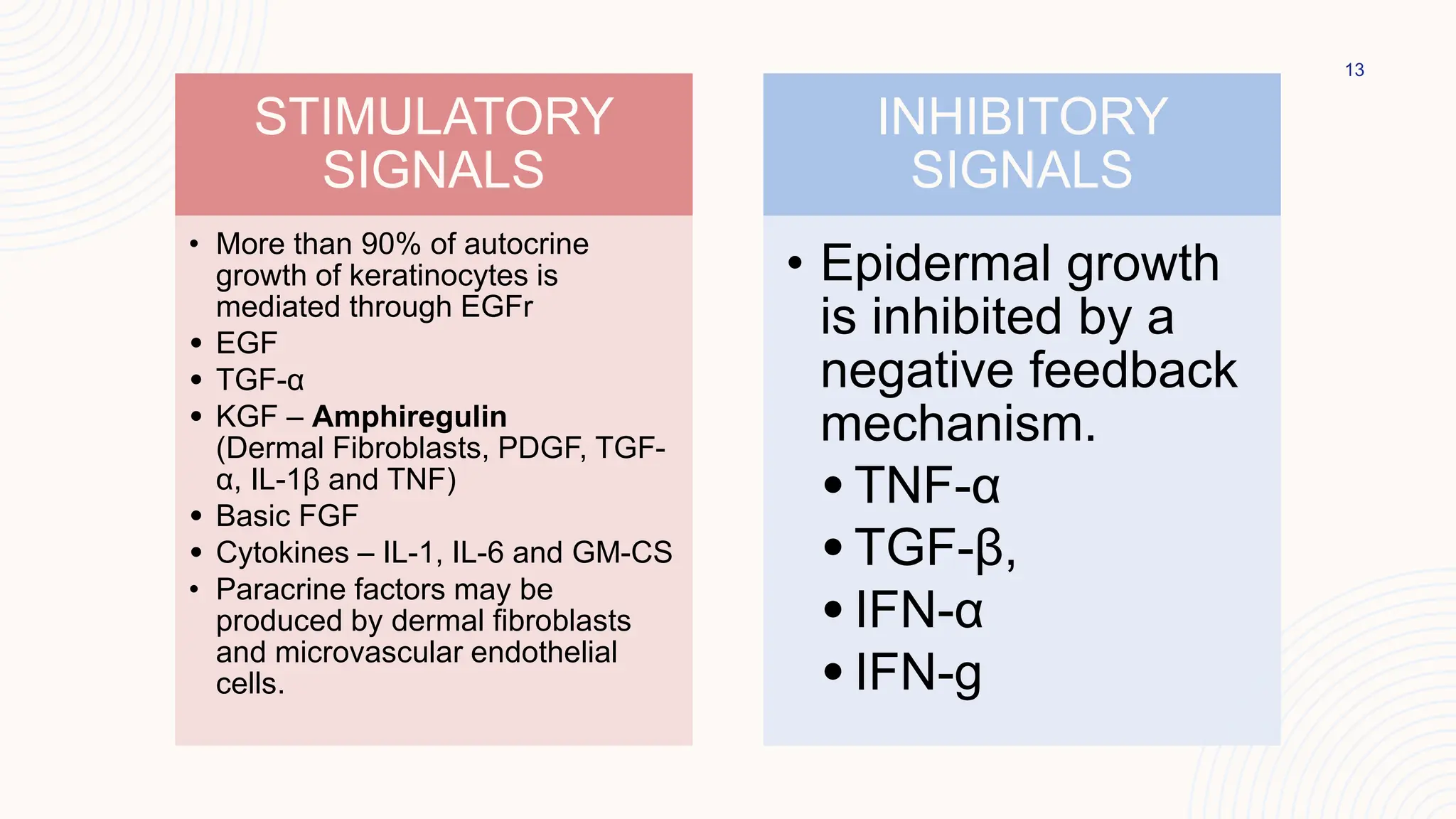
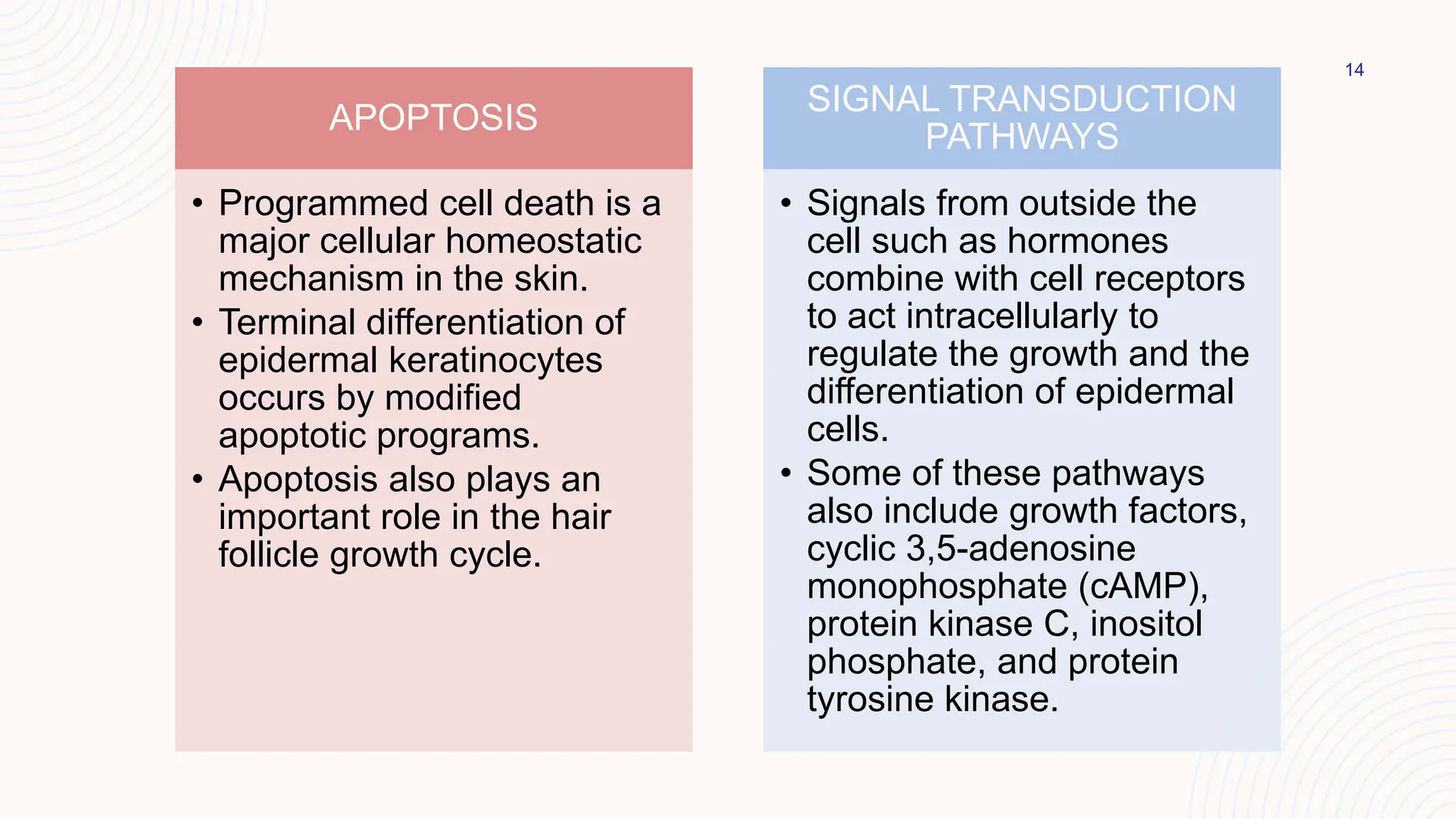
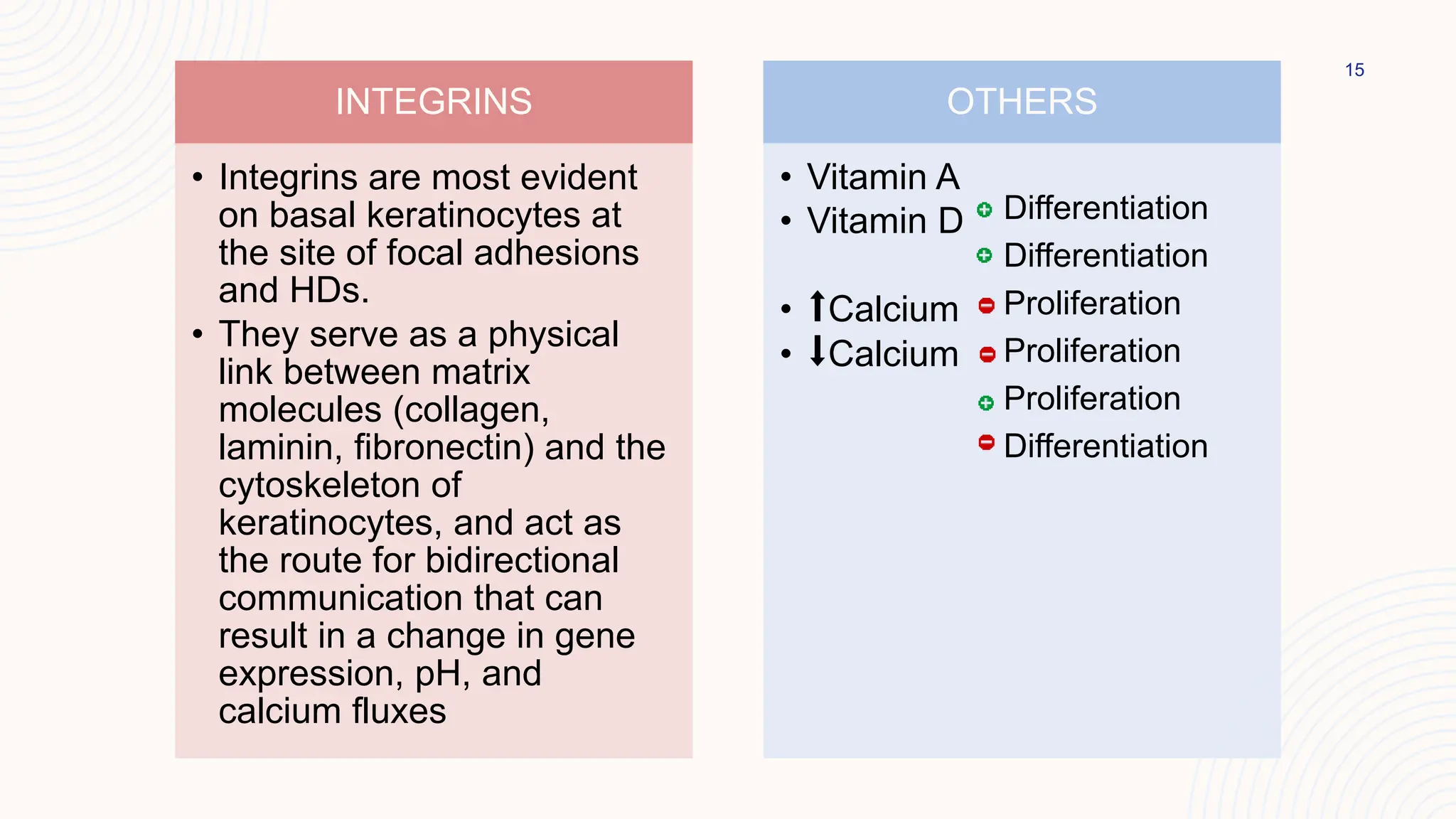
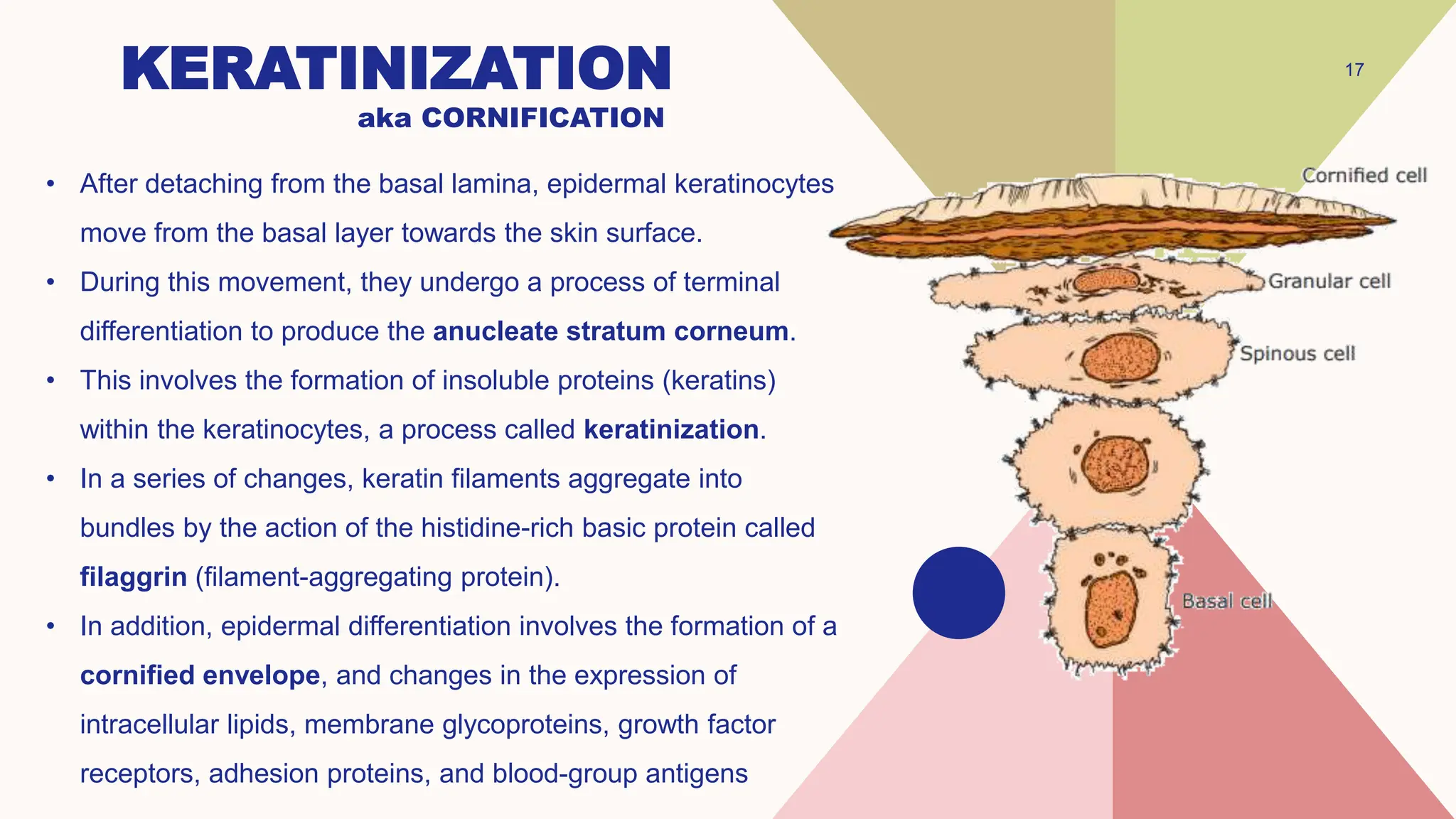
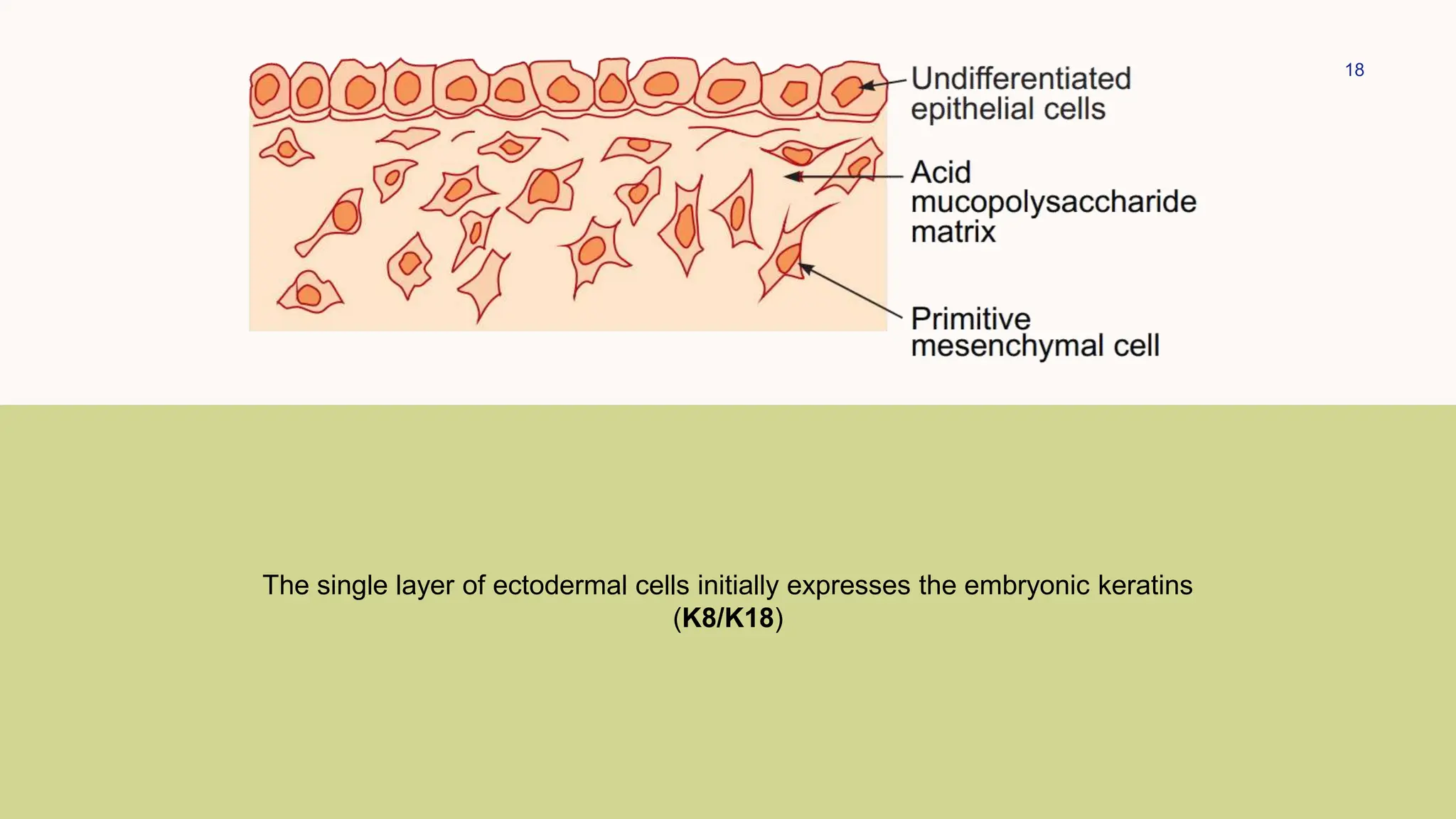
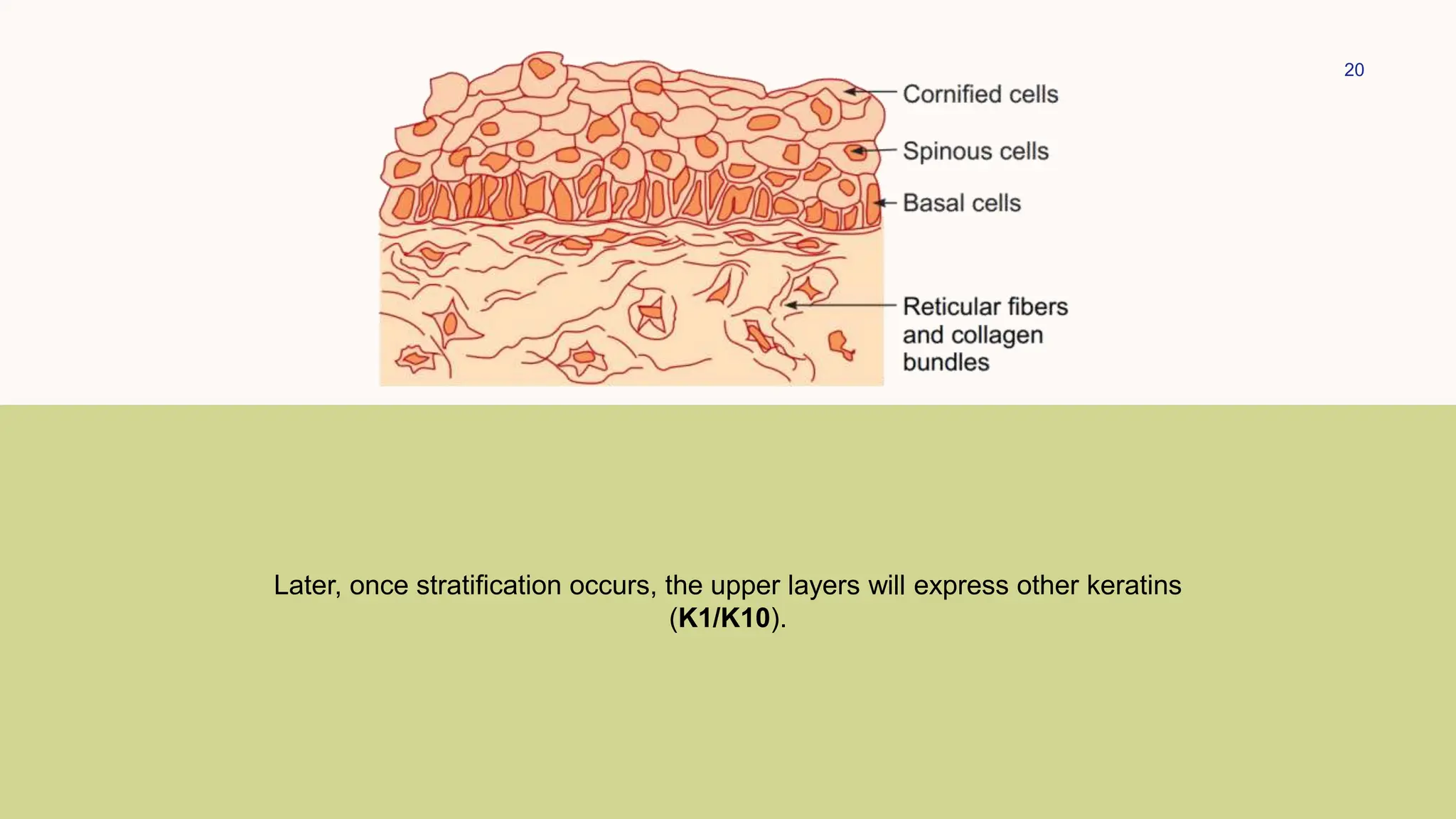
This document summarizes the development and kinetics of the epidermis. The epidermis originates from the ectoderm and develops in stages from a single cell layer to stratified squamous epithelium through embryogenesis. Its renewal is regulated through a balance of stimulatory and inhibitory signals that control the proliferation and differentiation of keratinocytes from the basal layer outward. As keratinocytes differentiate and migrate toward the skin surface, they undergo keratinization through the formation of keratins and other proteins that ultimately form the protective cornified layer.