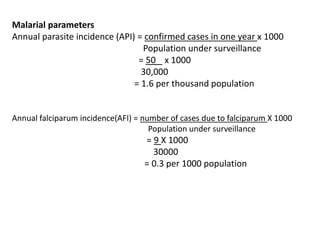
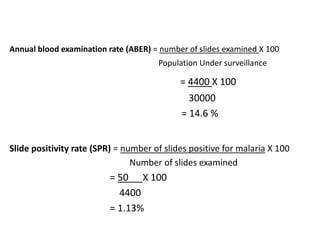
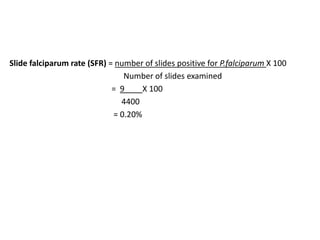
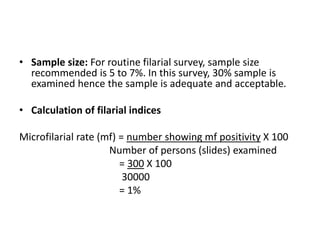
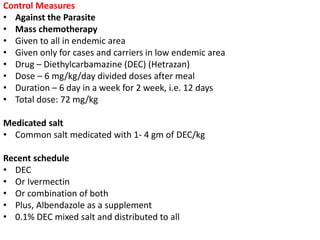
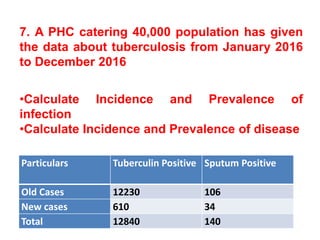
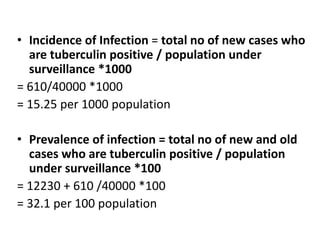
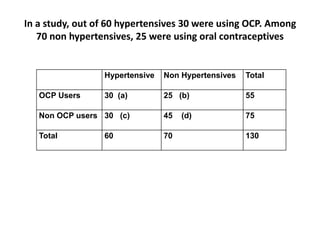
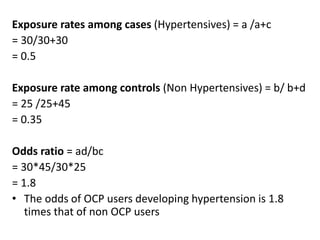
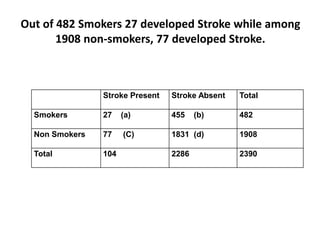
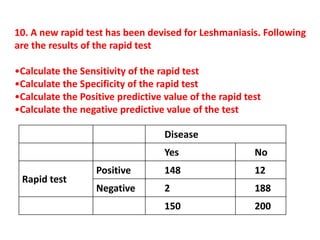
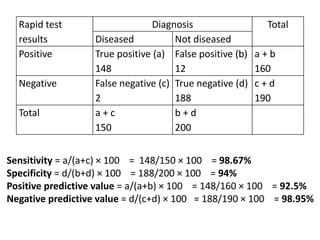
The document details the methodology and calculations for determining the required amount of bleaching powder to disinfect various water sources, including wells and swimming pools, using Horrocks test. It also discusses malaria surveillance and control measures in a specific health center, as well as handling a case of HIV in a pregnant woman. Furthermore, it provides statistical analysis and preventive strategies for filariasis and tuberculosis within designated populations.