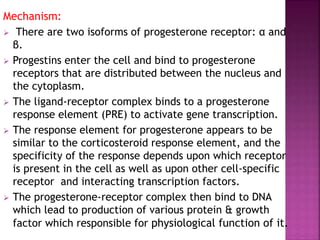
The document summarizes estrogens and progesterone. It discusses the three major estrogens - estradiol, estriol, and estrone. It describes estrogen levels in men and women throughout the menstrual cycle and after menopause. It also outlines the sites of estrogen production, mechanisms of action, physiological effects, routes of administration, uses, and adverse effects of estrogens. Selective estrogen receptor modulators (SERMs) like tamoxifen are also mentioned.