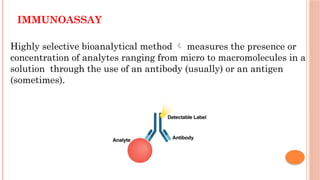
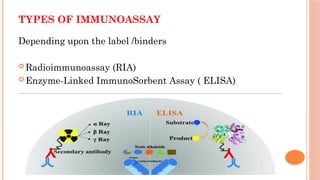
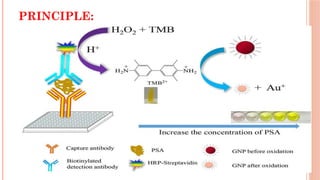
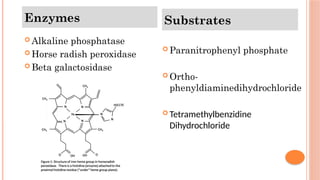
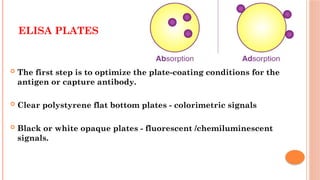
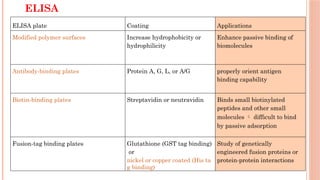
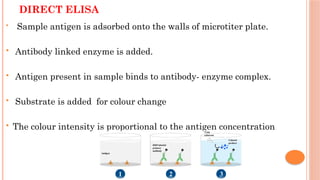
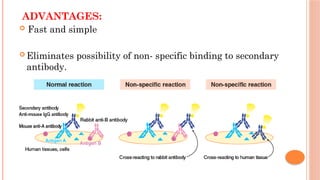
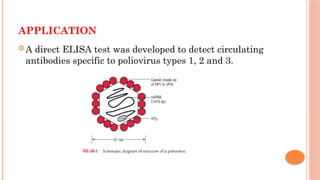
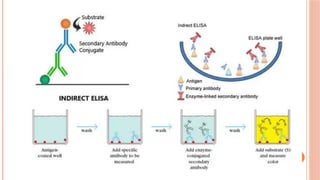
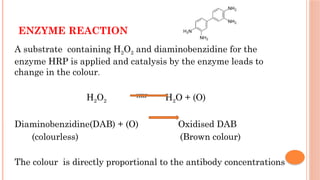
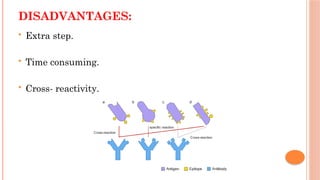
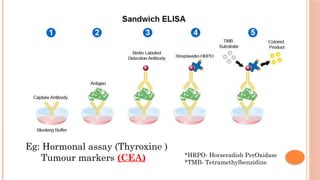
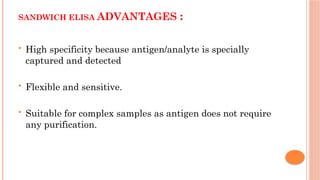
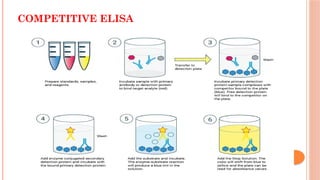
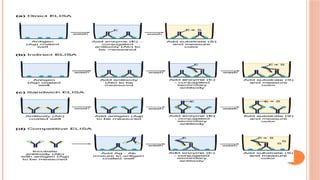
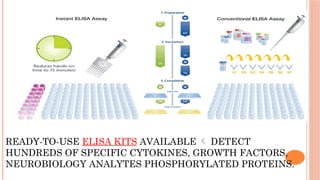
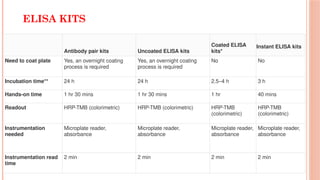
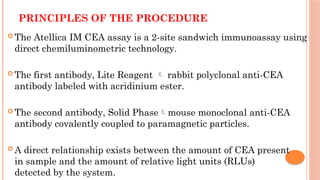
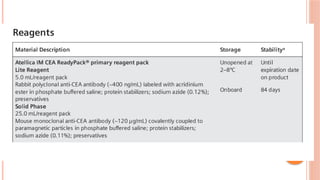
The document provides an overview of enzyme-linked immunosorbent assay (ELISA), detailing its principles, types, and applications in measuring analytes through antigen-antibody interactions. It describes various ELISA types such as direct, indirect, sandwich, and competitive ELISA, along with their respective advantages and disadvantages. Additionally, it explores the use of primary antibodies and detection strategies for quantifying results, emphasizing the role of ELISA in clinical research and disease monitoring.