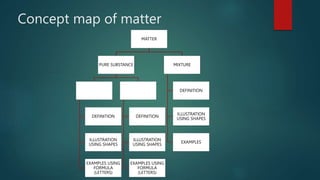
This document discusses classifying substances as elements or compounds. It provides definitions of elements, compounds, and mixtures. Elements are composed of only one type of atom and cannot be broken down further, while compounds are made of two or more types of atoms bonded together. Mixtures are made of two or more elements or compounds physically combined. The document outlines an activity where students will sort symbol cards into elements and compounds based on these definitions. They will then define elements and compounds based on the sorting rule.