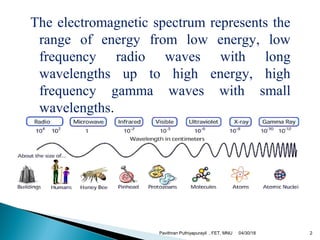
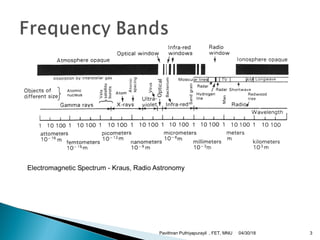
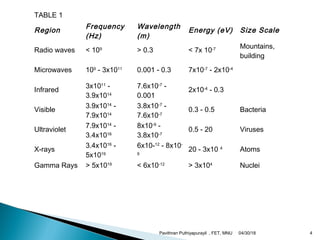
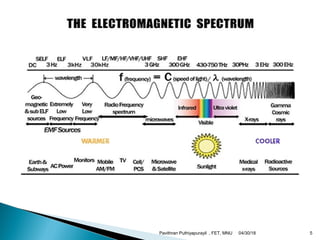
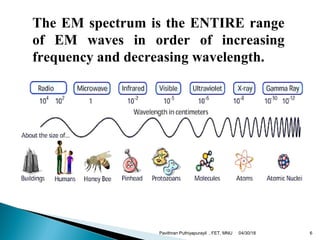
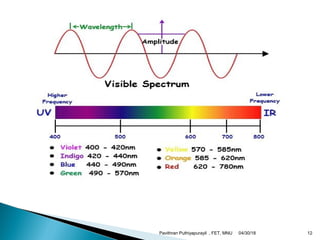
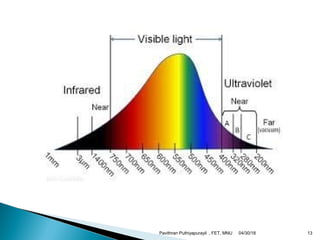
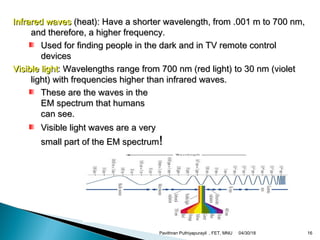
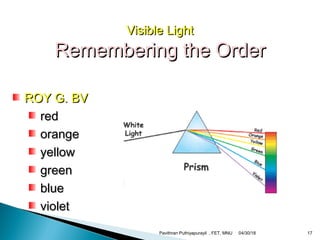
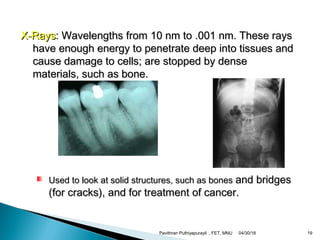
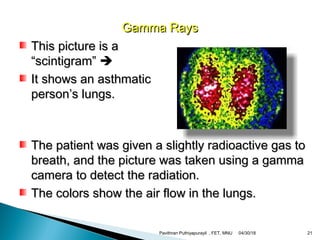
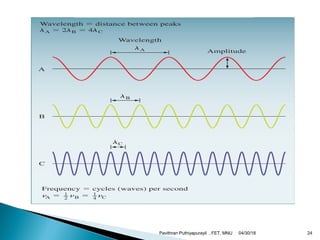
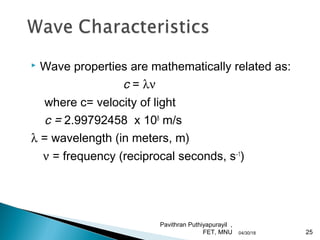
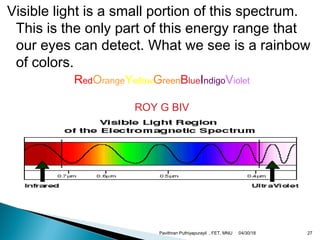
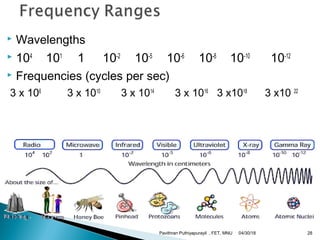
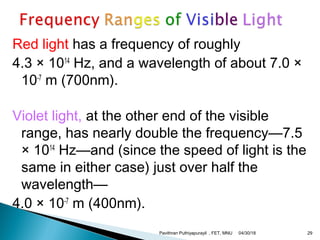
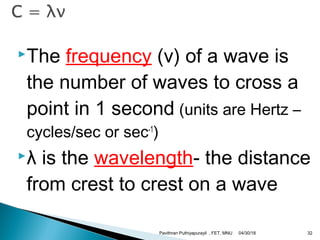
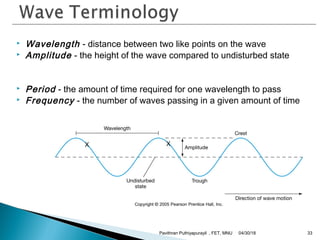
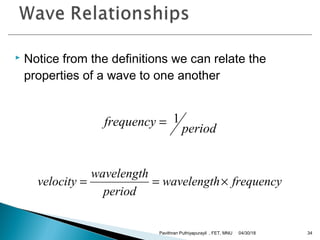
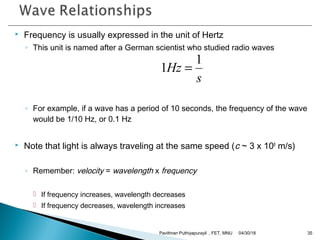
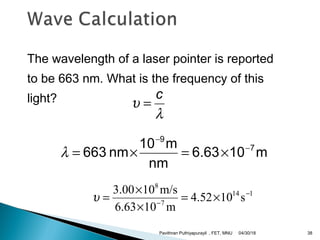
The electromagnetic spectrum represents the range of electromagnetic radiation from low energy, long wavelength radio waves to high energy, short wavelength gamma rays. It includes radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays and gamma rays. The document provides details on the wavelengths, frequencies and typical uses of different types of electromagnetic waves, including definitions of standard names for radio bands and common names for different frequency ranges used for communication technologies.