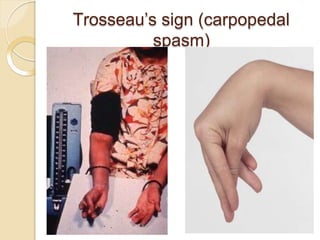
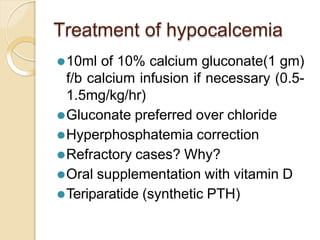
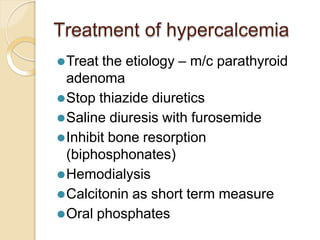
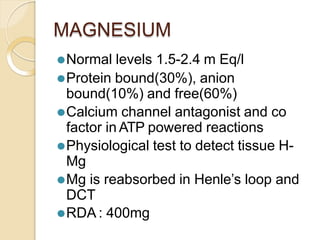
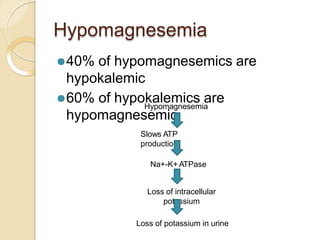
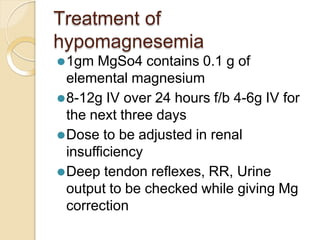
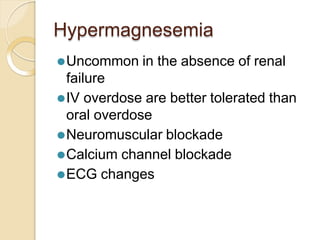
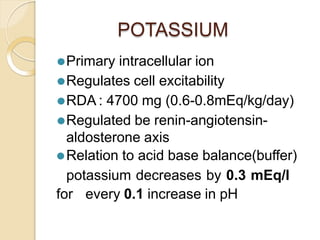
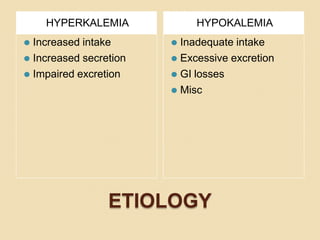
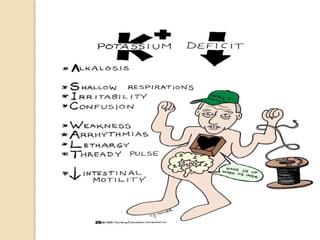
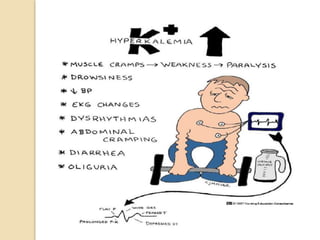
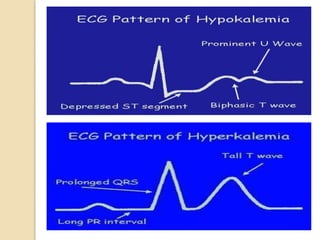
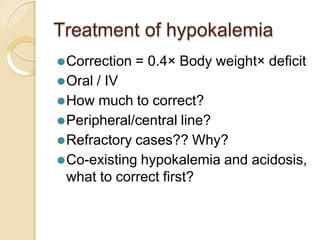
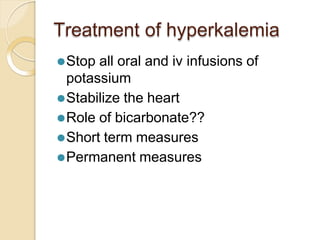
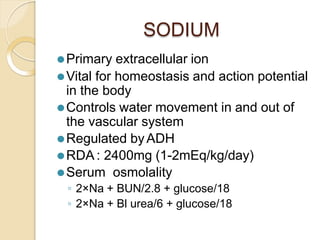
This document discusses electrolyte composition and imbalances in the body. It provides tables showing normal electrolyte levels in extracellular fluid, intracellular fluid, and gastrointestinal secretions. It then discusses potassium, sodium, calcium, and magnesium in more detail, including their roles, regulation, causes of imbalance, signs and symptoms, and treatment approaches for hypo- and hyper- conditions. Treatment sections focus on correction rates and addressing underlying causes, organ dysfunction, or excess intake/losses as appropriate for each electrolyte imbalance.

![CALCIUM
⚫Regulated by PTH and Calcitonin
⚫Vitamin D plays a role in absorption
⚫Coagulation cascade, neuromuscular
function
⚫Ionic 50%, protein bound 40%, anion
bound 10%
⚫RDA: 1-2g
⚫Ionic ca = total ca + [0.8×(4.5-albumin)]
⚫Relation to acid-base balance
◦ Acidosis decreases protein bound ca levels](https://image.slidesharecdn.com/electrolyteimbalance-220921094703-cc9ddd50/85/electrolyteimbalance-pptx-19-320.jpg)