EDTA_hardness.pptx
- 1. Water Purification: Hardness Estimation by EDTA method and its Removal using Ion-exchange Resin
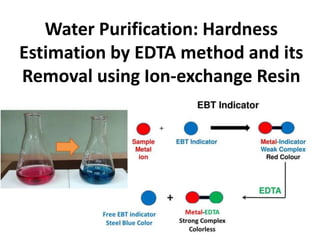
- 2. Expt. No.: Date: Experiment Water Purification: Hardness Estimation by EDTA method and its Removal using Ion-exchange Resin Problem definition Hardness of water is due to the presence of dissolved calcium and magnesium salts in water. EDTA forms stable complex with hardness causing salts and is used in the removal of scale and sludge forming impurities in industrial boilers. Methodology EBT indicator-Metal ion complex is weaker compared to EDTA-metal ion complex. The end point is the color change from wine red (EBT- Metal ion complex) to steel blue (free EBT indicator). Solution Estimation of Calcium hardness (in ppm) in the given unknown sample. Understanding the water softening using ion-exchange resins. Student learning outcomes (SLO) Students will learn to a) perform complexometric titration b) understand the efficiency of ion-exchange resins using in water purifiers
- 3. 1). Introduction to Hard Water and its Classification: Hard water contains high levels of dissolved Ca2+and Mg2+ions. Ground and surface water dissolve the Ca2+/Mg2+ containing ores/minerals from surrounding soil and rock and the solution gets enriched with these cations. Hardness is commonly expressed as mg of CaCO3 eq./L. Amount of hardness causing chemicals: 60 mg/l = Soft water: 60–120 mg/l = Moderately hard water. 120–180 mg/l = Hard water. >180 mg/l = Very hard water. Based on the type of anions (Cl-, SO4 2-, HCO3 -) associated with Ca2+/Mg2+ ions, the hardness is categorized into temporary (carbonate, HCO3 -) hardness & permanent (non-carbonate, Cl-, SO4 2-) hardness.
- 4. 2). Problems caused by Hard Water: Hard water can cause costly breakdowns in boilers and plumbing. When hard water is heated, the hardness causing salts tend to precipitate out of solution, forming hard scale or soft sludge in pipes and surfaces, thereby restricting water flow. Scale formation in boilers prevents efficient heat transfer, resulting in energy loss and overheating leading to serious accidents. At the domestic level, hard water lessens the effectiveness of soap by forming scums/precipitates, which adhere to human skin. Human consumption of water containing excess of Ca and Mg are associated with increased risks of osteoporosis, colorectal cancer, hypertension, stroke, coronary artery disease, diarrhea and obesity.
- 5. 3). Modern Treatment of Hard Water: Hard water can be softened using ion- exchange resins (IER). IERs are very small, porous polymeric beads with sulphonic/carboxylic acid/Na+ functional groups attached to the polymer backbone. When hard water is passed through the IER beads, Ca2+/Mg2+ ions in hard water are trapped by the IER and hydrogen/Na+ ions are released, thereby softening the hard water. Exhausted IER beads (saturated with Ca2+/Mg2+ ions) are regenerated using mild acid/brine solution to flush out Ca2+/Mg2+ ions. The regenerated IER can be reused for many cycles.
- 6. 4). Principle: Ehtylenediaminetetraacetic acid (EDTA) forms complexes with a large number of cations including Ca2+ and Mg2+ depending upon pH of solution. Since EDTA is insoluble in water, the disodium salt of EDTA is used. The resulting metal-ligand complex, in which EDTA forms a cage-like structure around the metal ion, is very stable at specific pH. EDTA Disodium salt of EDTA Metal-EDTA complex
- 7. Hardness in water can be estimated by complexometric titration using sodium salt of EDTA and EBT indicator at pH = 9-10. EBT forms an wine-red colored weak/unstable complex with Ca2+/Mg2+ions, which upon titrating with EDTA, results in the breaking of EBT-Ca2+/Mg2+ unstable bond and formation of stable/strong EDTA-Ca2+/Mg2+ bond. The endpoint changes from wine-red (EBT-Ca2+/Mg2+) to steel blue (free EBT indicator).
- 8. 5). Reagents and solutions: Standard hard water (SHW, 1mg/mL of CaCO3 equivalents), 0.01 N EDTA solution, Eriochrome Black – T (EBT) indicator, hard water sample, NH3-NH4Cl buffer solution and ion exchange resin. 6). Apparatus: Burette, pipette, conical flask, standard flask, IER column. 7). Procedure: (i). Titration-1: Standardization of EDTA Pipette out 20 mL of SHW containing 1mg/mL of CaCO3 (1000 ppm) into a clean conical flask. Add 1 T.T. full of buffer solution to maintain the pH around 10. Add 3 drops of EBT indicator and titrate it against the given EDTA solution taken in the burette. End point is change of colour from wine red to steel blue. Repeat the titration for concordant titer values. Let ‘V1’ be the volume of EDTA consumed.
- 9. Calculation: 20 mL of given hard water consumes V1 mL of EDTA 20 mg of CaCO3 requires V1 mL of EDTA for complexation 1 mL of EDTA requires = 20/V1 mg CaCO3 for complexation This relation will be used in other two titrations S. No. Volume of standard hard water (mL) Burette reading (mL) Volume of EDTA (V1, mL) Initial Final 1. 20 ml 0 19.8 19.8 ml 2. 20 ml 0 19.8 19.8 ml 3. Concordant titer value
- 10. (ii). Titration-2: Estimation of total hardness of hard water sample Pipette out 20 mL of the given sample of hard water into a clean conical flask. Add 1 T.T. of buffer solution and 3 drops of EBT indicator. Titrate this mixture against standardized EDTA solution taken in the burette. The end point is the change of color from wine red to steel blue. Repeat the titration for concordant titer value. Let ‘V2’ be the volume of EDTA consumed. S. No. Volume of sample hard water (mL) Burette reading (mL) Volume of EDTA (V2, mL) Initial Final 1. 20 ml 0 11.3 11.3 ml 2. 20 ml 0 11.3 11.3 ml 3. Concordant titer value Calculation: 20 mL of sample hard water consumes = V2 mL of EDTA. = V2 x 20/V1 mg of CaCO3 eq. 1000 mL of hard water sample consumes = V2 x 20/V1×1000/20 = V2/V1×1000 ppm Total hardness of the water sample = “X” ppm
- 11. (iii). Titration-3: Removal of hardness using ion exchange method Ion exchange is a reversible process. When hard water is passed through cation ion-exchange resins (IERs) packed in a narrow column, Ca2+ and Mg2+ cations in hard water are exchanged with Na+ or H+ ions in IERs. The exhausted resins are regenerated by passing 10% dil. HCl through the column. Applications of IERs: preparation of high-purity water for power engineering, electronic and nuclear industries, in household water purifiers.
- 12. Methodology: Arrange the IER column to burette stand and pour the hard water sample (around 40 to 50 mL) remaining after the completion of Titration – 2 into IER column. Collect the water passing through the column in a beaker over a period of 10 min. Adjust the valve of the column to match the duration of outflow. From the water collected through the column, pipette out 20 mL into a clean conical flask and repeat the EDTA titration. Volume of EDTA consumed as ‘V3’. S. No. Volume of sample hard water (mL) Burette reading (mL) Volume of EDTA (V3, mL) Initial Final 1. 20 ml 0 0.6 0.6 ml 2. 20 ml 0 0.6 0.6 ml 3. Concordant titer value Calculation: 20 mL of water sample after passing through IER consumes V3 mL of EDTA. = V3 x 20/V1 mg of CaCO3 eq. 1000 mL of water sample after softening through IER column consumes = V3 x 20/V1×1000/20 = V3/V1×1000 ppm Residual hardness of the water sample = “Y” ppm
- 13. 8). Final Results: Total hardness of the water sample= …………….(X) ppm Residual hardness in the water sample= ……………(Y) ppm Hardness removed through the column = …………….(X–Y) ppm 9). Link for video presentation: https://youtu.be/wkfK98OnrHE V1 = 19.8 ml V2 = 11.3 ml V3 = 0.6 ml