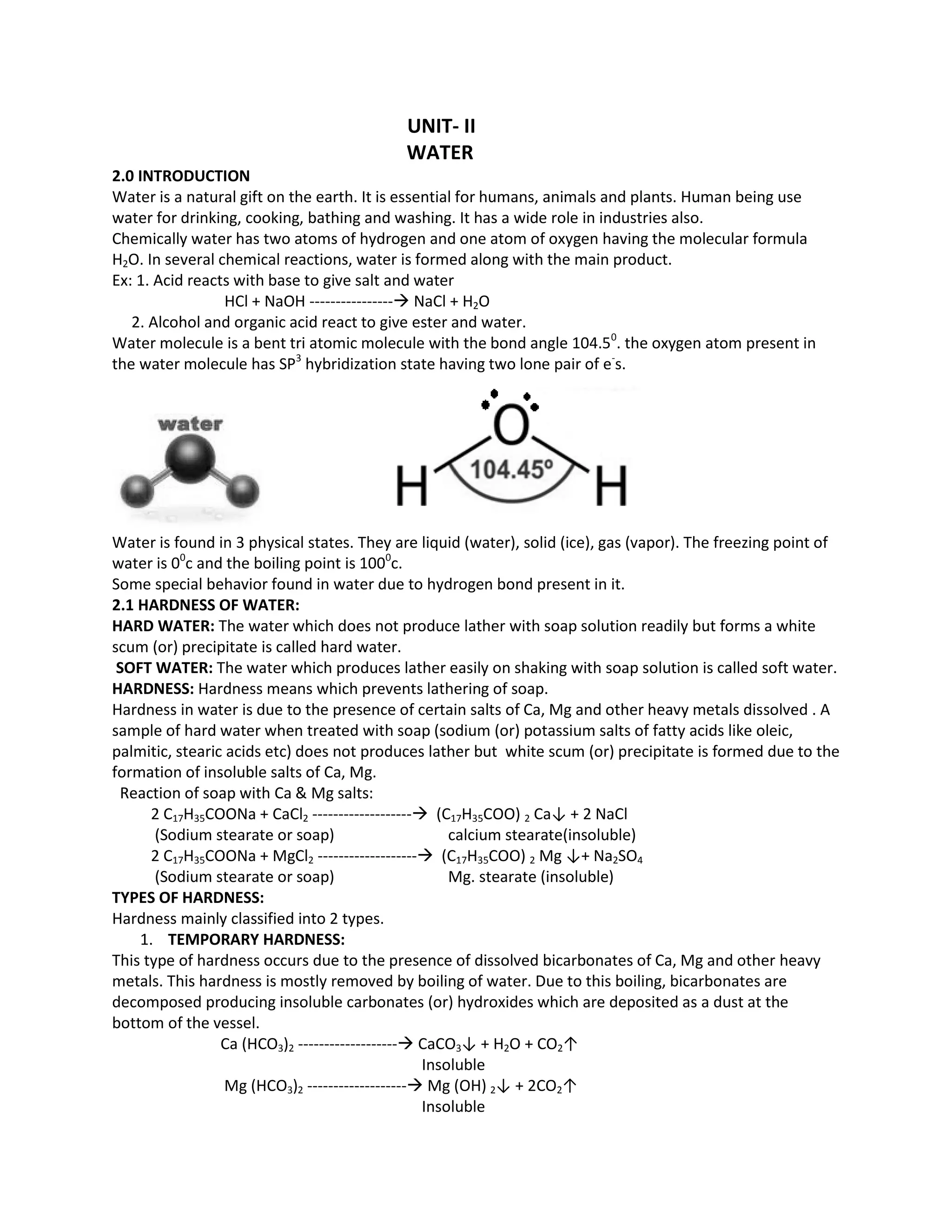
Water is essential for humans, animals and plants. It is used for drinking, cooking, bathing and washing. Water also plays an important role in industries. Hardness in water is caused by dissolved salts of calcium, magnesium and other metals. This prevents soap from lathering easily. Hardness can be classified as temporary, caused by bicarbonates, or permanent, caused by chlorides and sulfates. The EDTA method is commonly used to determine water hardness by forming complexes with calcium and magnesium ions. Scale and sludge formation in boilers occurs when salt concentrations exceed solubility limits during steam production, potentially weakening boiler walls.
![2. PERMANENT HARDNESS:
This type of hardness occurs due to the presence of chlorides, sulphides of Ca, Mg, Fe and other heavy
metals. This hardness cannot be removed by boiling like in temporary hardness.
UNITS OF HARDNESS: Generally the hardness is expressed in terms of equivalent amount of CaCO3.
Parts per million (PPM): It is the parts of CaCO3 equivalent hardness per 106
parts of water.
1 PPM = 1 part of CaCO3 equivalent hardness in 106
parts of water.
Milligrams per liter (mg/lit): Number of milligrams of CaCO3 equivalent hardness present per liter of
water.
1 mg/l =1mg of CaCO3 equivalent hardness in 1 lit of water
1 lit of water weight, 1kg =1000g =1000 Χ1000 = 106
mg
1 mg/lit = 1mg of CaCO3 Equivalent hardness per 106
mg of water
= 1part of CaCO3 Equivalent hardness per 106
parts of water =1 PPM
Degree Clarkes (0
Cl) : Number of Grains of CaCO3 Equivalent Hardness per Gallon of Water
1 grain = 68.4 mg
1 gallon = 3.78541lit ≈ 4 lit
It is part of CaCO3 equivalent hardness per 70,000 parts of water.
10
Clarke = 1 grain of CaCO3 equivalent hardness per gallon of water.
= 1 part of CaCO3 equivalent hardness per 70000 parts of water.
Degree French (0
Fr): It is the parts of CaCO3equivalent hardness per 105
parts of water.
10
Fr = 1 part of CaCO3 equivalent hardness per 105
parts of water.
RELATIONSHIP BETWEEN VARIOUS UNITS OF HARDNESS:
1PPM = 1 mg/lit = 0.10
Fr = 0.02 meq/lit
2.2 DETERMINATION OF HARDNESS OF WATER
ESTIMATION OF HARDNESS:
The estimation of hardness of water is very essential for its use in boilers for steam generation as well as
Industrial uses.
EDTA METHOD:
Di sodium salt of Ethylene Diamine Tetra Acetic acid (EDTA) is used as the permanent complexing agent
with the Ca2+
& Mg2+
ions of hard water in the EDTA method.
STRUCTURE OF EDTA:
Before starting the titration to the hard water, ammonia buffer (to maintain pH = 9 - 10) and Eriochrome
Black – T indicator are added, which forms an unstable complex of wine red coloured.
Ca2+
+ EBT ---------------- [Ca - EBT]
Blue unstable, wine red coloured complex
Mg2+
+ EBT ---------------- [Mg - EBT]
Blue unstable, wine red coloured complex
After the titration, the sodium salt of EDTA forms stable complex with water containing Ca2+
& Mg2+
ions
replacing the unstable complex.](https://image.slidesharecdn.com/water-171018131749/85/Water-by-aluru-jaideep-reddy-2-320.jpg)
![The completion of the complex formation is indicated by Eriochrome black – T indicator at a pH range
9 – 10 giving a blue colour solution.
[Ca - EBT] ------------------- [Ca - EDTA] + EBT
Unstable stable, colourless blue
[Mg - EBT] ---------------- [Mg - EDTA] + EBT
Unstable stable, colourless blue
STANDARDIZATION OF EDTA:
20ml of standard hard water (standard ZnSO4 solution) is pipette out into a clean conical flask; add 4 – 5
ml buffer solution & 2 – 3 drops of EBT indicator. The wine red colour solution in the conical flask is
titrated with EDTA till pale blue colour appears which indicates the end point of the titration.
ZnSO4 EDTA
M1V1/n1 = M2V2/n2
M1 = molarity of hard water (zinc sulphate)
V1 = volume of hard water (zinc sulphate)
M2 = molarity of EDTA
V2 = volume of EDTA
ESTIMATION OF TOTAL HARDNESS:
50 ml of sample hard water is in clean conical flask and add 4 – 5 ml of buffer & 2 – 3 drops of EBT
indicator. The wine red colour solution in the conical flask is titrated with EDTA till pale blue colour
appears which indicates the end point of the titration.
Total hardness =
C = volume of EDTA (burette reading).
D = 1 ml 0.01 M EDTA = 1 mg of CaCO3
1 ml -- ---M EDTA =?
ESTIMATION OF PERMANENT HARDNESS:
Take 100 ml of water sample into a beaker and boil the water till the volume reduces to 50 ml. filter the
solution and add the 4 -5 ml of buffer solution and 2 – 3 drops of EBT indicator. The wine red colour
solution in the conical flask is titrated with EDTA till pale blue colour appears which indicates the end
point of the titration.
TOTAL HARDNESS =
C = volume of EDTA (burette reading).
D = 1 ml 0.01 M EDTA = 1 mg of CaCO3
1 ml -- ---M EDTA =?
Temporary hardness = total hardness – permanent hardness
SOAP TITRATION METHOD:
Hardness of water is determined by this method without using any indicator. Known volume of water
sample is taken and titrated against soap solution (standard). Initially, lather is not formed due to the
hardness but at point lather is formed which persists for 2 minutes. By this method total hardness of
water is measured.
On boiling the known volume of water sample for halfenhour, temporary hardness removed as
precipitate of (Ca2+
/Mg2+
) carbonates. This sample is further titrated to find the permanent hardness.
Temporary Hardness = Total Hardness – Permanent Hardness
The standard soap solution of soap can be obtained from the market or otherwise it can be prepared
and then standardized in the laboratory with standard CaCl2 solution.
2 C17H35COONa + Ca2+
------------------- (C17H35COO) 2Ca↓+2 Na+
Soap calcium stearate](https://image.slidesharecdn.com/water-171018131749/85/Water-by-aluru-jaideep-reddy-3-320.jpg)

![Scales are hard deposits formed by the evaporation of hard water in boilers. Due to the scale formation
in boilers more amount of heat has to be supplies for heating since the scales produced on the walls of
boilers acts as insulators of heat.
Due to the overheating of the boiler certain areas of the boiler weaken causing distortion and even lead
to breaking in high pressure boilers.
Some of the salts mainly responsible for scale formation are CaSiO3, CaSO4 and Mg (OH)2.
Ca (HCO3)2 present in hard water decomposes at higher temperature producing CaCO3.
Ca (HCO3)2 CaCO3 + H2O + CO2
CaCO3 and Mg (OH)2 can form loose sludges as well as scales.
These are mainly formed as scales in low pressure boilers.
Calcium sulphate get deposited in the boilers as scales since the solubility of the salt decreases with
increase in temperature. CaSO4 get deposited on the heated position of the boiler.
Mg(OH)2 is produces in the boiler by the hydrolysis of MgCl2 salt.
MgCl2 + H2O Mg (OH)2 + 2HCl
CaSiO3 and SiO2 can form very hard scales in turbine blades due to their tendency to mix with the steam
produces. The silica content can be removed by the solution of very small amount of mgO to the
permanent hard water (after the removal CaCO3 precipitate) which produces magnesia silica sludge that
can be removed easily. By using small amount of ferrous sulphate or sodium aluminate coagulants, silica
can be covered with the colloidal Al(OH)3 or Fe(OH)2 and the colloidal sludge can be easily removed from
the boilers.
REMOVAL OF SCALES:
Scales can be removed by applying thermal shocks (sudden heating and cooling).
Using scrapers, wire brush etc., scales can be removed.
Using certain chemicals scales can be removed.
Ex: using 5 – 10% HCl, CaCO3 scales can be removed.
Using EDTA, CaSO4 scales can be removed.
By “blow down operation” (removing the bottom portion of salt concentrated water of the boiler) the
scales formation can be avoided
2.4 INTERNAL TREATMENT OF BOILER FEED WATER
PREVENTION OF SCALE FORMATION BY INTERNAL TREATMENT:
The following are the internal conditioning methods used in boilers:
1. CARBONATE CONDITIONING:
In low pressure boilers scale formation can be avoided by treating the boiler water with sodium
carbonate. The scale forming salts like CaSO4 are partially removed.
CaSO4 + Na2CO3 CaCO3 + Na2SO4
CaCO3 is precipitated in the boiler as loose sludge which can be scraped off. For the precipitation of
CaCO3 the carbonate ions added should exceed the sulphate ions present in the water.
2. COLLOIDAL CONDITIONING:
Scale formation in boilers is mainly due to crystalline precipitate. When certain chemicals like tannin or
agar gel are added to water, these substances get coated on the outer surface of crystalline precipitates
and forms colloidal, non sticky and sludge like precipitates which can be easily removed by mechanical
methods of blow down operation.
3. CALGON CONDITIONING :
In this process calgon or sodium hexa meta phosphate [Na(PO3)]6 is added to the boiler feed water
which forms soluble complex with the CaSO4 scales.
Na2 [Na4 (PO3)6] 2Na+
+ [Na4 (PO3)6]2-
2 CaSO4 + [Na4 (PO3)6]2-
[Ca2 (PO3)6]2-
+ 2Na2SO4
Soluble complex](https://image.slidesharecdn.com/water-171018131749/85/Water-by-aluru-jaideep-reddy-5-320.jpg)

![H2SO4 + Ca (OH)2 CaSO4 + 2H2O
CO2 + Ca (OH)2 CaCO3 + H2O
It precipitates the bicarbonates of calcium and magnesium as carbonates.
Ca (HCO3)2 + Ca (OH)2 2CaCO3 +2H2O
Mg (HCO3)2 + Ca(OH)2 2CaCO3 + Mg(OH)2 +2H2O
It precipitates the bicarbonate ions (NaHCO3/KHCO3) into carbonates.
2NaHCO3 + Ca (OH)2 CaCO3 + 2H2O + Na2CO3
It can react only with magnesium permanent hard salts (MgCl2 and MgSO4) producing precipitates.
MgCl2 + Ca (OH)2 Mg (OH)2 + CaCl2
MgSO4 + Ca (OH)2 Mg (OH)2 + CaSO4
It also precipitates the iron and aluminium salts
FeSO4 + Ca (OH)2 Fe (OH)2 + CaSo4
Al2 (SO4)3 +3Ca (OH)2 Al (OH)3 + 3 CaSo4
Neglecting traces of iron and aluminum salts present in water and considering the other salts and their
reaction with lime we can see that only Mg(HCO3)3 consumes 2 ca(OH)2 i.e. double the amount of lime.
Hence the final equation for the quantitative requirement of lime for the above mentioned hard salt is
given below
Lime required =
74
100
[Temp Ca 2+
+2Temp Mg 2+
+perm(Mg2+
+Fe2+
+Al3+
) +CO2 +
1
2
HCl +H2SO4 +
1
2
HCO3
-
]
all in terms of CaCO3 eq
Molecular weight of lime = 74
Molecular weight of CaCO3 = 100
The following are the chemical reactions involving soda with the hardness producing salts (or) functions
of soda.
Neutralization of free acids
2HCl + Na2CO3 2NaCl + H2O + CO2
H2SO4 + Na2CO3 Na2SO4 + H2O + CO2
Precipitation in permanent hard salts of calcium and magnesium
CaCl2+ Na2CO3 CaCO3 + 2NaCl
CaSO4 +Na2CO3 CaCO3 + Na2SO4
MgCl2+ Na2CO3 MgCO3 + 2NaCl
MgSO4 + Na2CO3 MgCO3 + Na2SO4
The sodium bicarbonate present in the hard water reacts with soda producing more sodium carbonate.
Hence in the formula for the quantitative requirement of soda the value of bicarbonate ions have to be
subtracted.
Soda requirement =
106
100
[perm (Ca 2+
+Mg2+
+Fe2+
)+3permAl3+
+
1
2
HCl +H2SO4 -
1
2
HCO3
-
]
all in terms of CaCO3 eq
Molecular weight of soda = 106
Molecular weight of CaCO3 = 100
The following are the methods adopted for the efficiency of the process.
Through stirring of chemicals and water using stirrers.
Proper time for completion of reaction.](https://image.slidesharecdn.com/water-171018131749/85/Water-by-aluru-jaideep-reddy-7-320.jpg)






