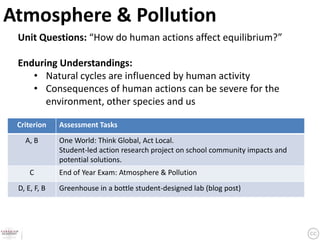
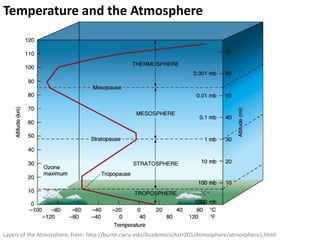
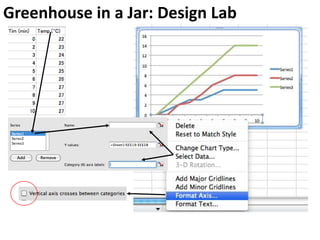
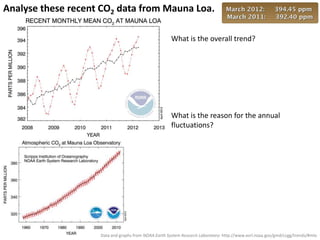
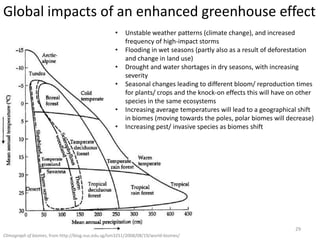
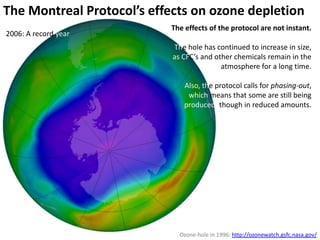
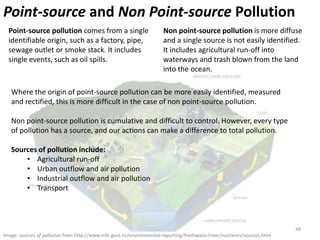
This document provides an overview of a unit on atmosphere and pollution from a Canadian environmental science academy. It includes unit questions, enduring understandings, assessment criteria, and topics to be covered such as the composition and layers of the atmosphere, the greenhouse effect, ozone layer, and air pollution. Students will conduct projects and exams to demonstrate their understanding of how human actions affect atmospheric equilibrium and the consequences for the environment.