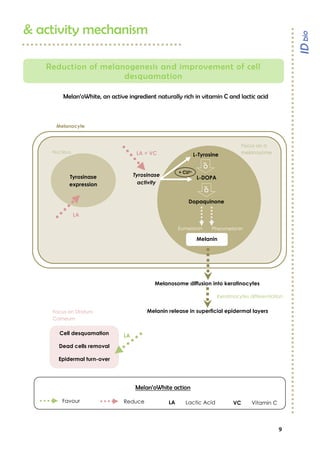
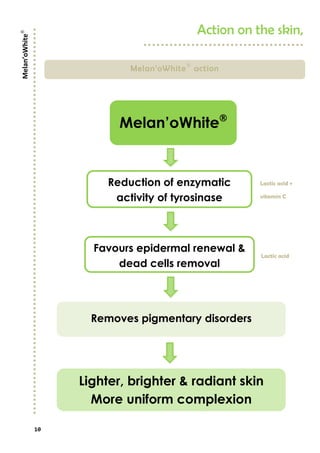
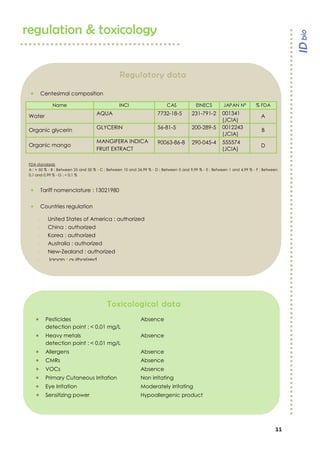
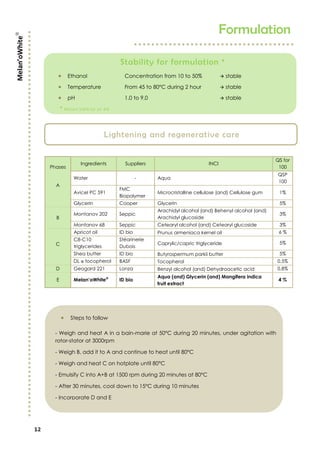
Melan’owhite® is a naturally derived active ingredient rich in lactic acid and vitamin C, designed to lighten skin and improve uneven pigmentation through its effects on tyrosinase activity and cell renewal. Clinical studies show it significantly reduces melanin synthesis and improves skin tone uniformity with daily use over 28 to 56 days. The product is eco-certified, preservative-free, and customizable for formulation needs.




![7
Placebo Melan’oWhite
a*
Δ Compared to T0 a* T28 a* T56 b* T28 b* T56
Placebo -1,2% -2,6% +0,5% +1,3%
Melan’oWhite -4,3% -2,6% +0,7% +1,0%
Efficacy measurement on lightening effect
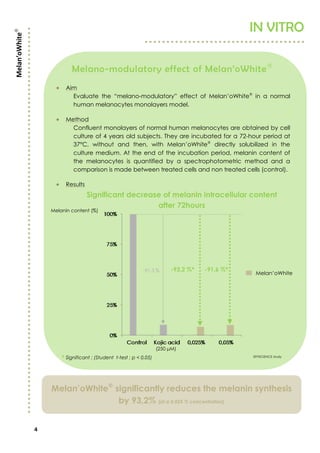
Aim
Evaluate and compare Melan’oWhite
lightening efficiency against
placebo.
Population
23 Asian female volunteers, aged from 18 to 60 years old, with all skin types.
Method
One daily application (in the evening) of a 4% Melan’oWhite
emulsion
and placebo emulsion, on randomized inner forearm during 28 and 56
days. At the end of both periods, parameters L*a*b and ITA° are measured
by chromametry. Results are given for L* and ITA° which give the best
description of a lightening effect.
L* = Luminance which represent the relative brightness from total darkness (L*=0) to absolute
white (L*100)
ITA° = Individual Typological Angle Pigmentation degree calculated with ITA° = Arctg [(L*-
50)/b*].(180/π) - Tanned skin ITA° < 27° whereas very light skin ITA° > 55°.
Results
Significant ITA° and L* increase vs placebo at T28 and T56 days
* Significant ; (Student t-test ; p < 0,01) SPINCONTROL study
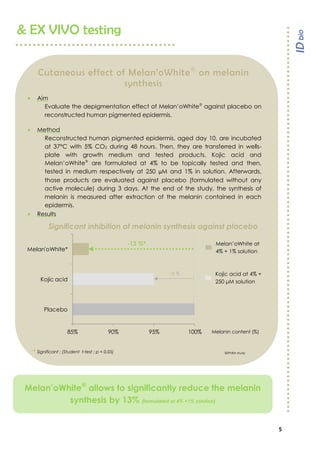
& IN VIVO results
Melan’oWhite
enables a significant lightening improvement
after 28 days (+4,6% for ITA°/+0,9% for L*) and 56 days (+6,7% for ITA°/+1,4% for L*)
with only 1 daily application
+6,7 %*
+1,2 %
+4,6 %*
-1,3 %
Variation compared
to T0 (%)
T28 T56
+1,4 %
+0,3 %
+0,9 %
-0,2%
-2%
0%
2%
4%
6%
8%
Placebo
Melan'oWhite
Variation compared
to T0 (%)
T28 T56
-1%
1%
2%
Placebo
Melan'oWhite
ITA° L*
-1,3%
+1,2%
+1,4 %*
+0,9 %*](https://image.slidesharecdn.com/dtmelanowhite-150312090108-conversion-gate01/85/Melan-oWhite-7-320.jpg)