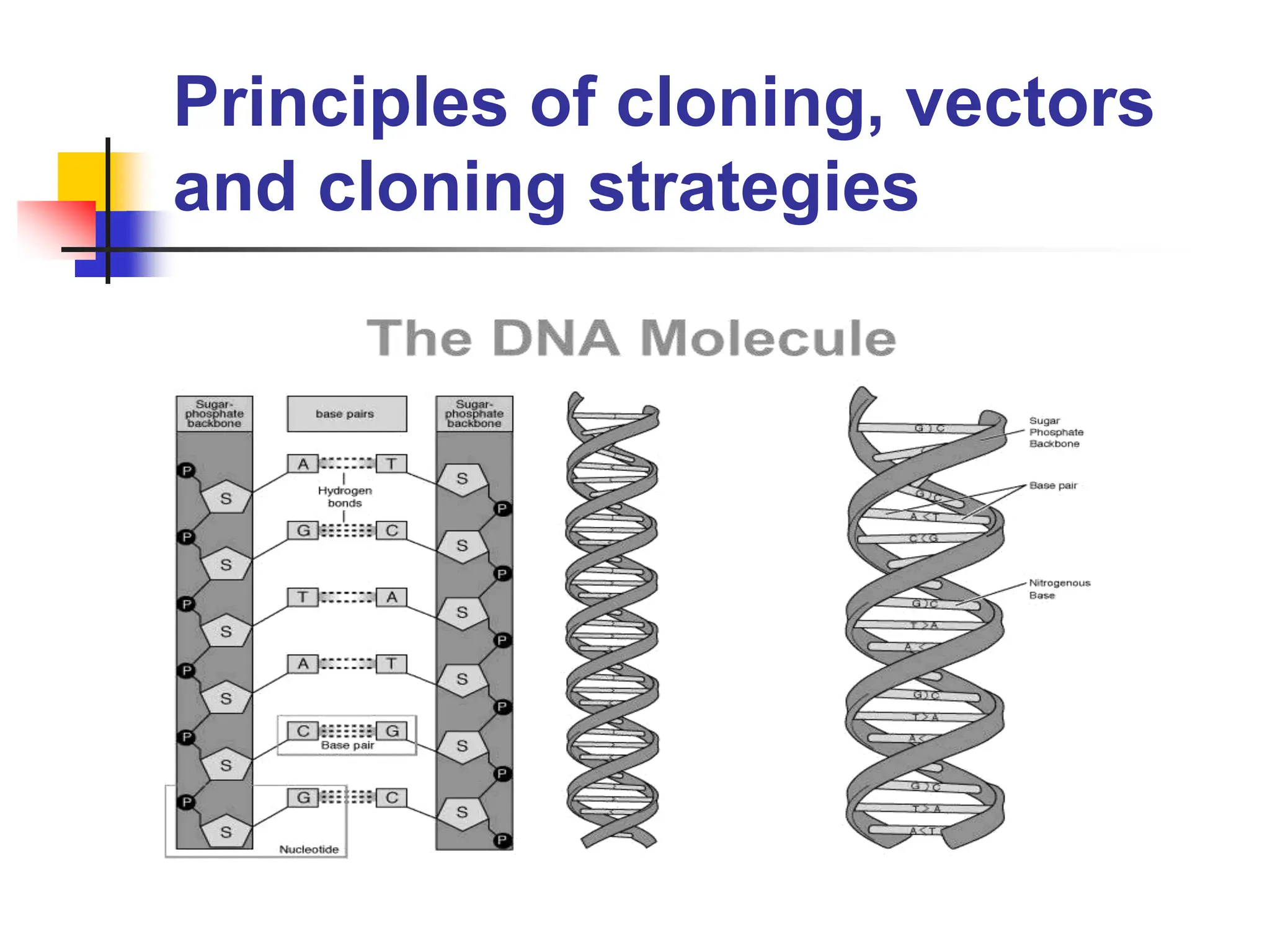
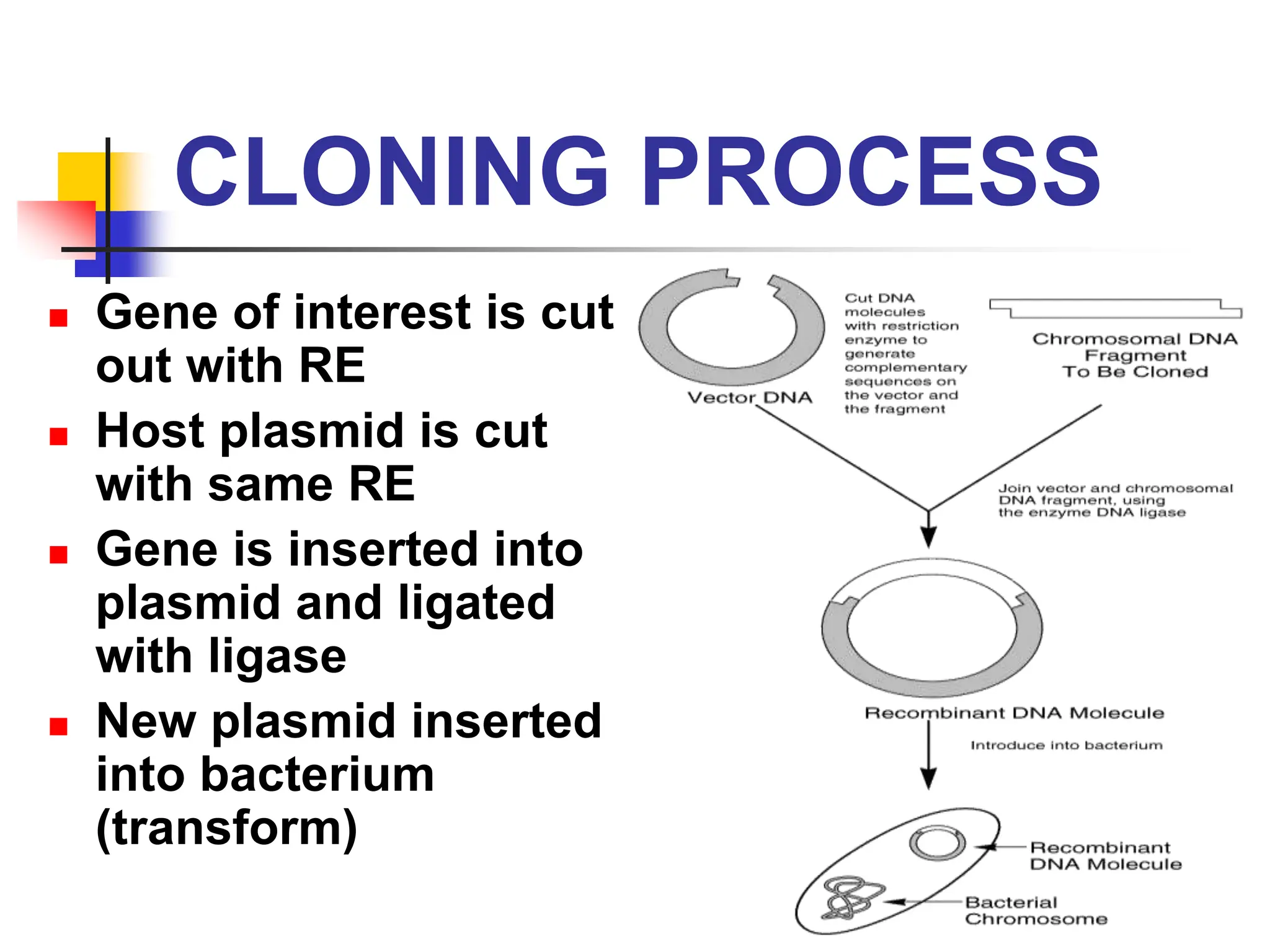
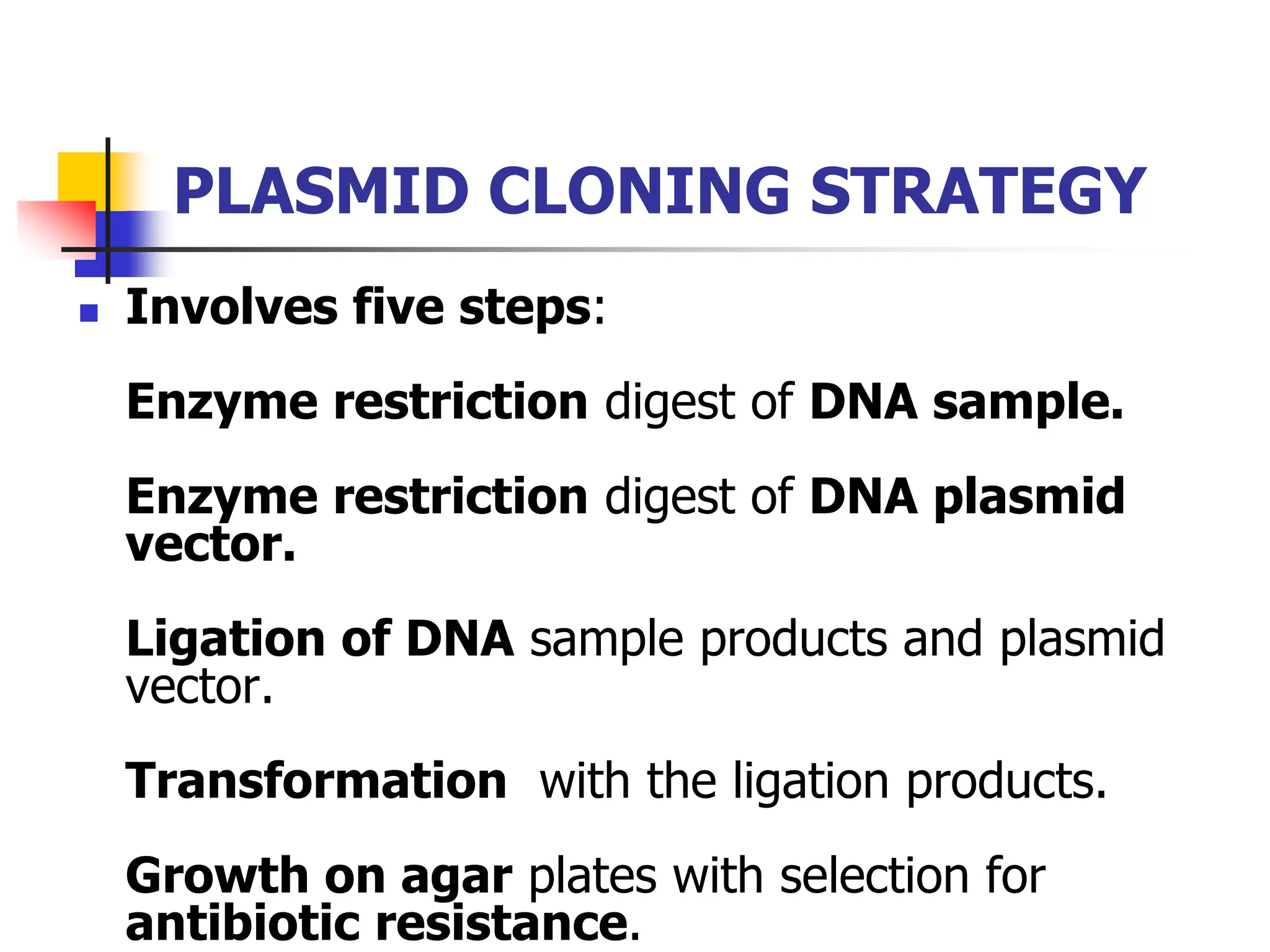
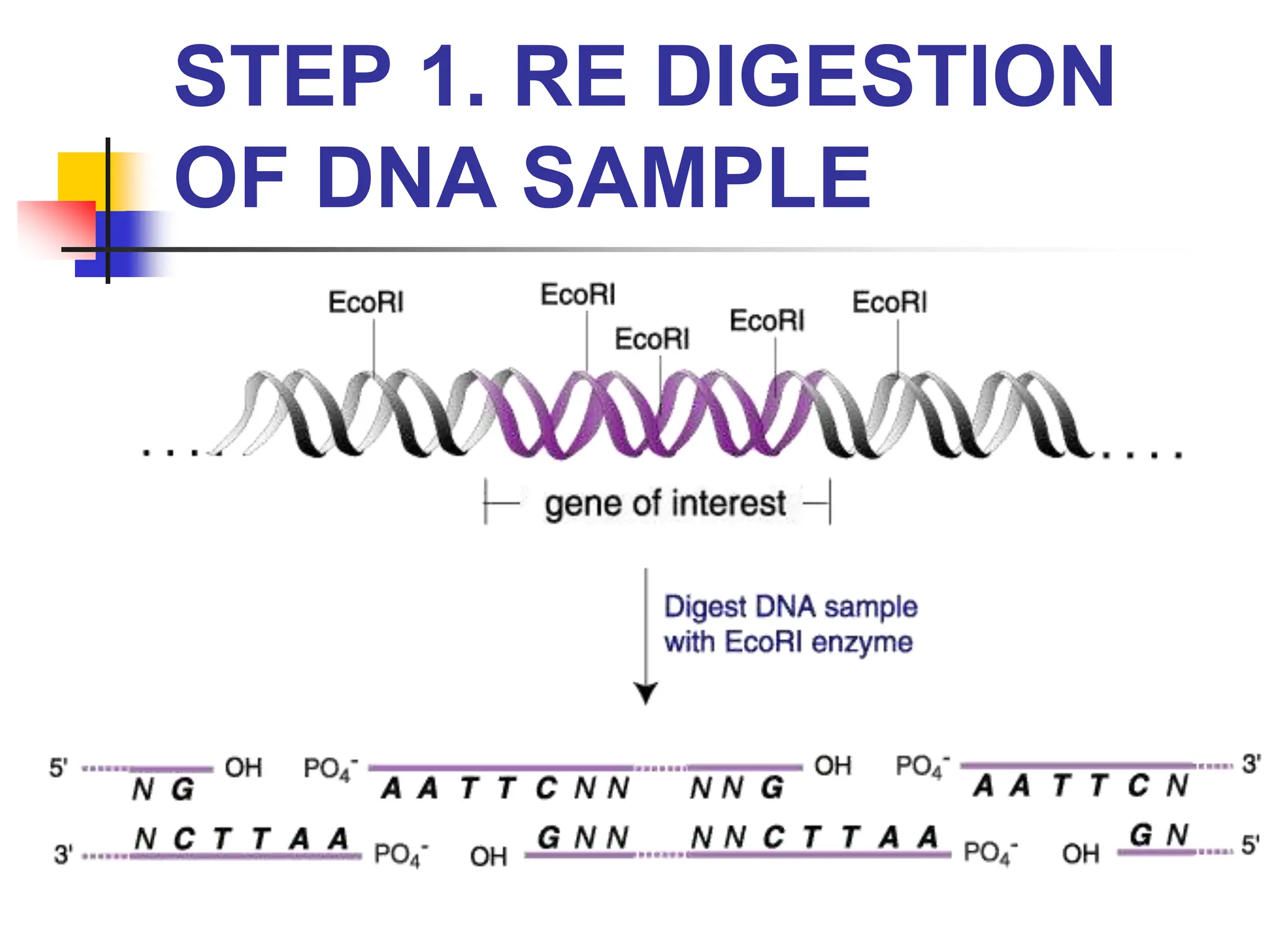
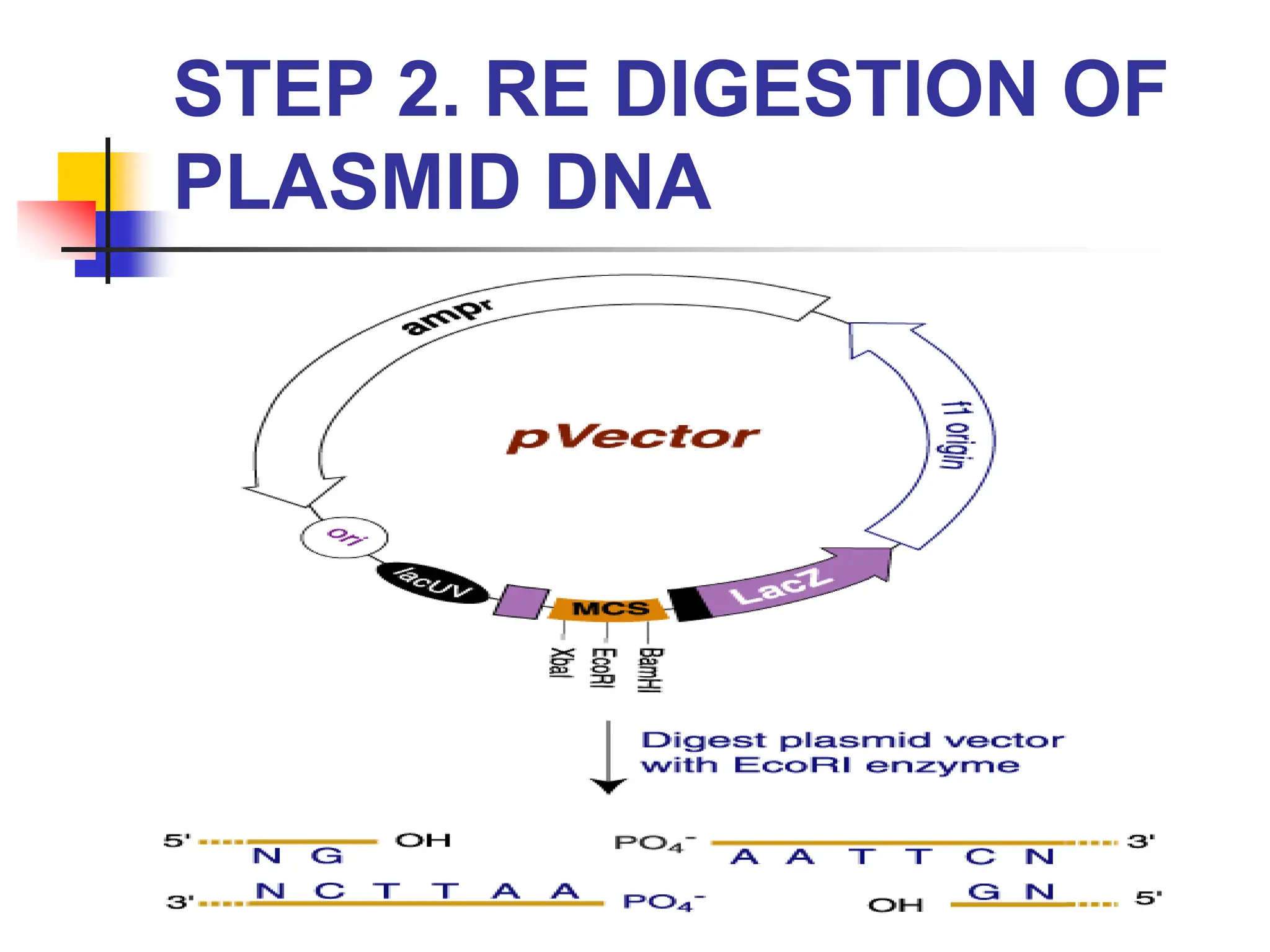
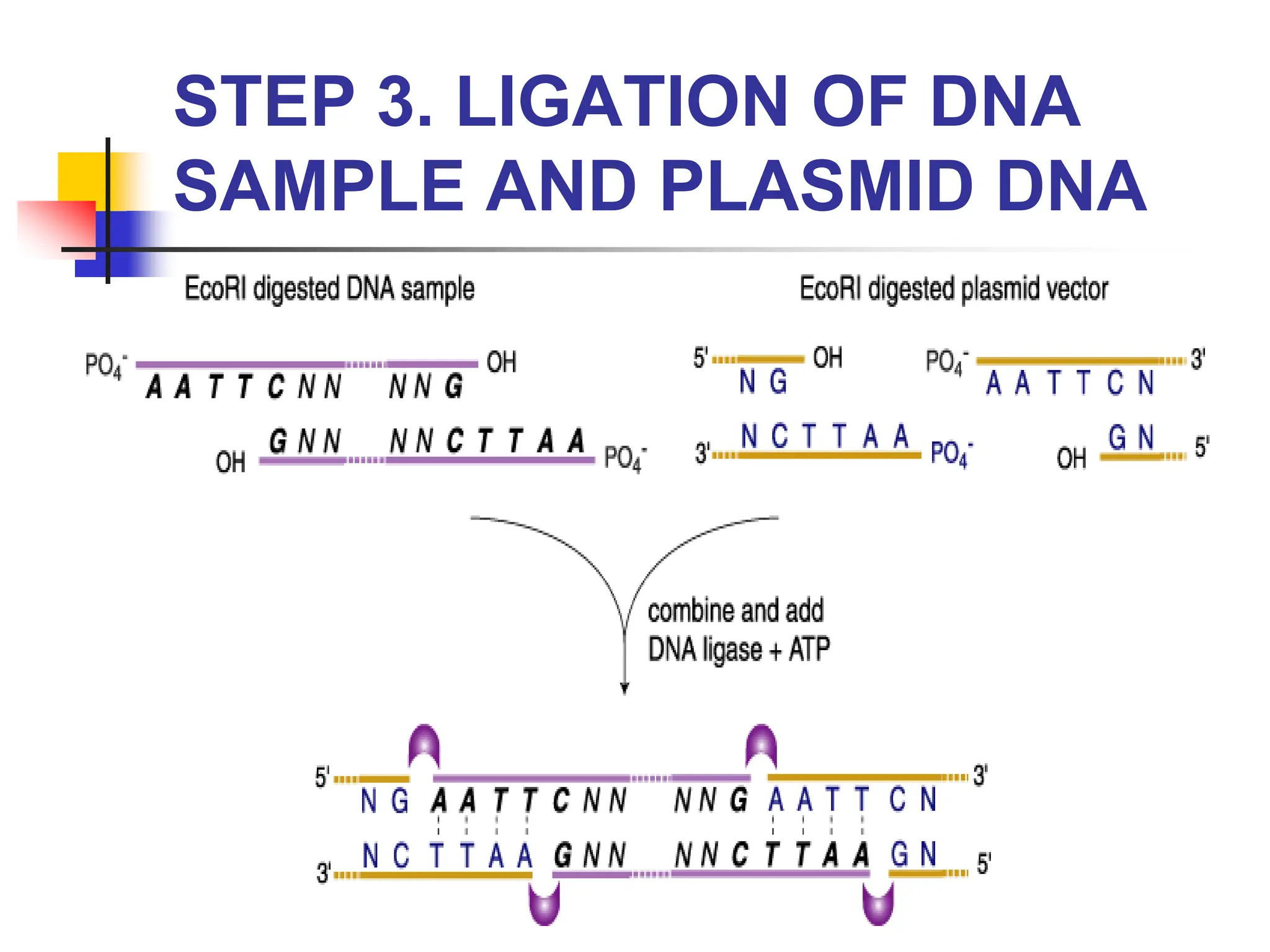
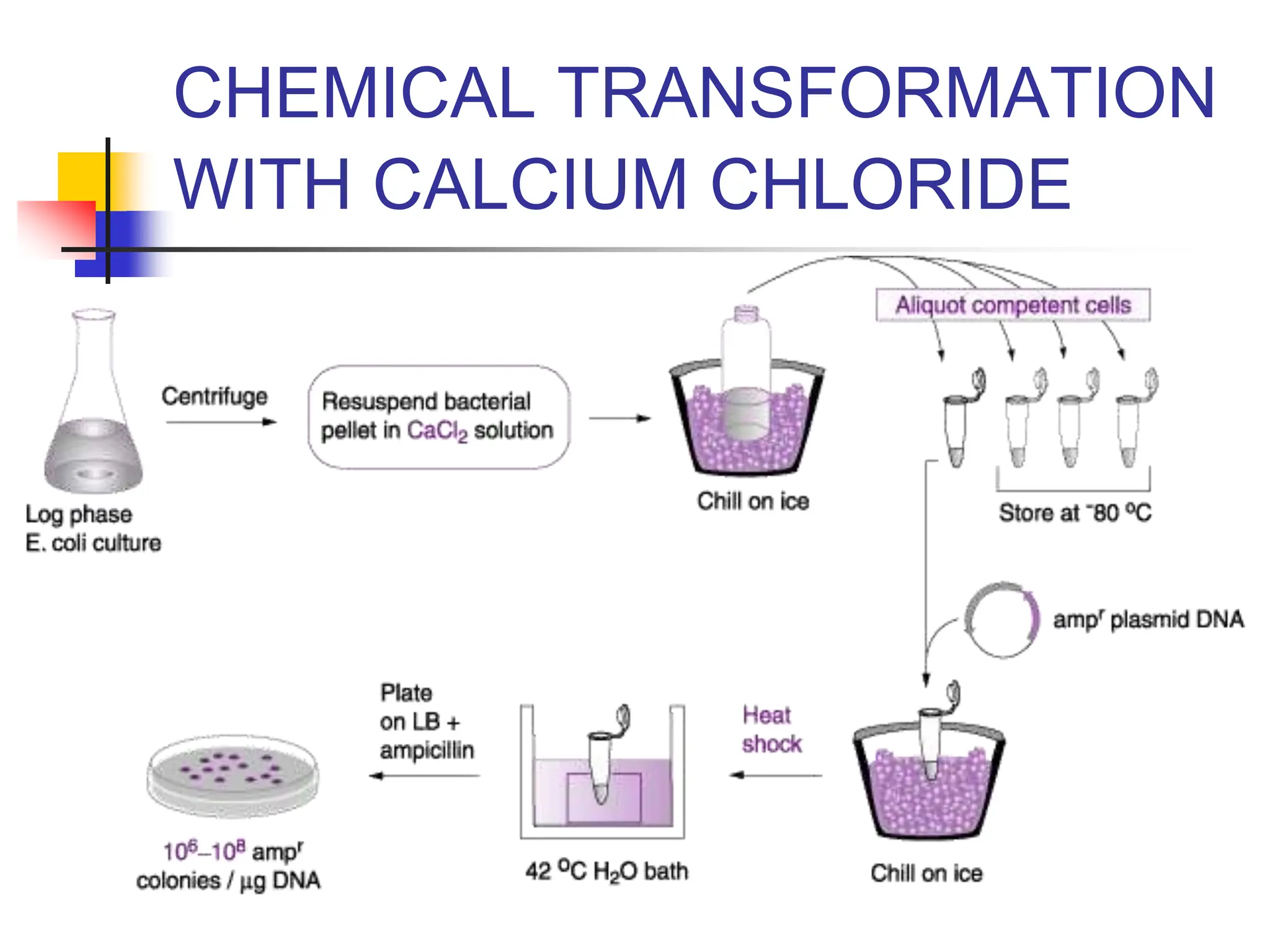
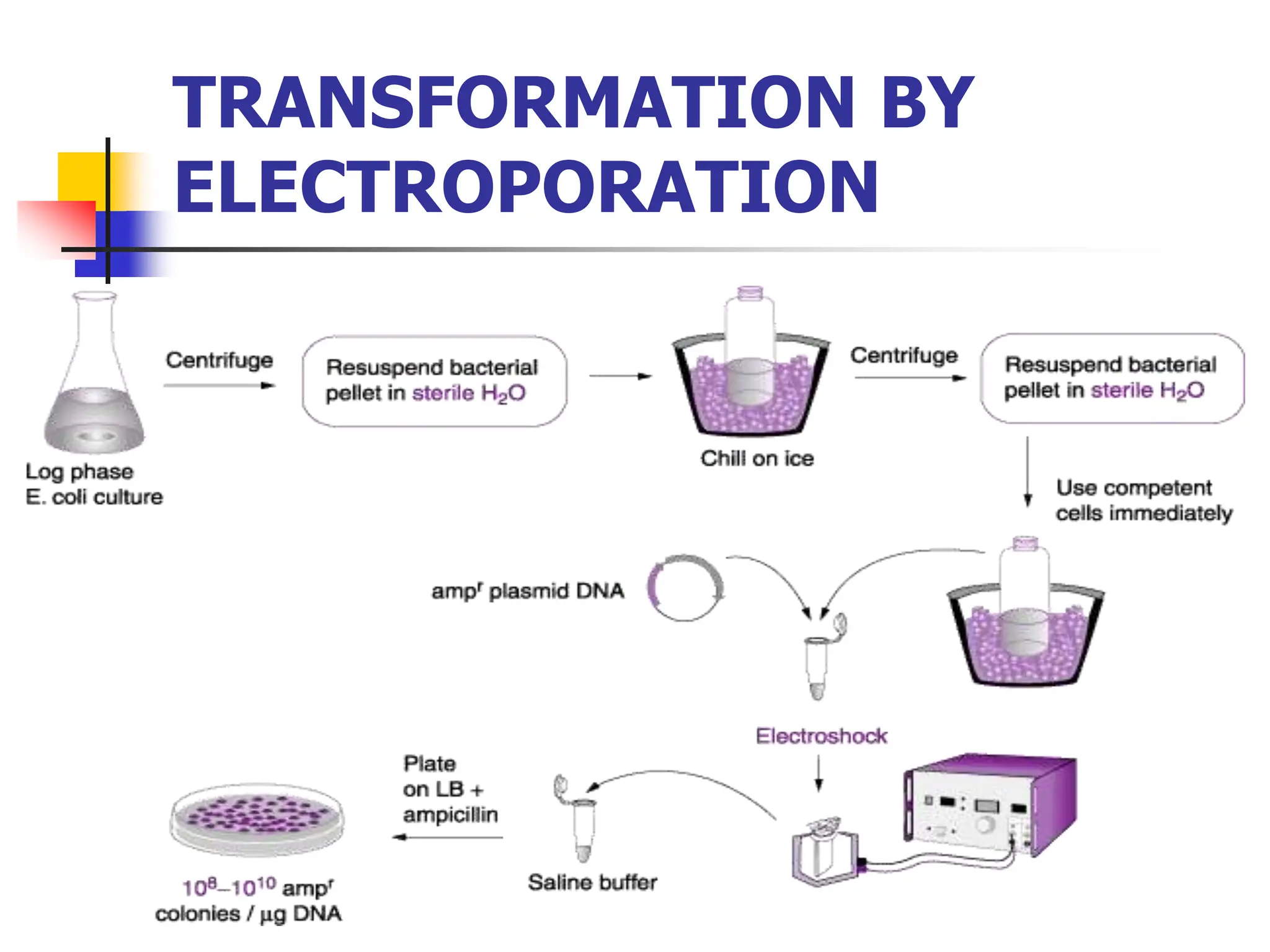
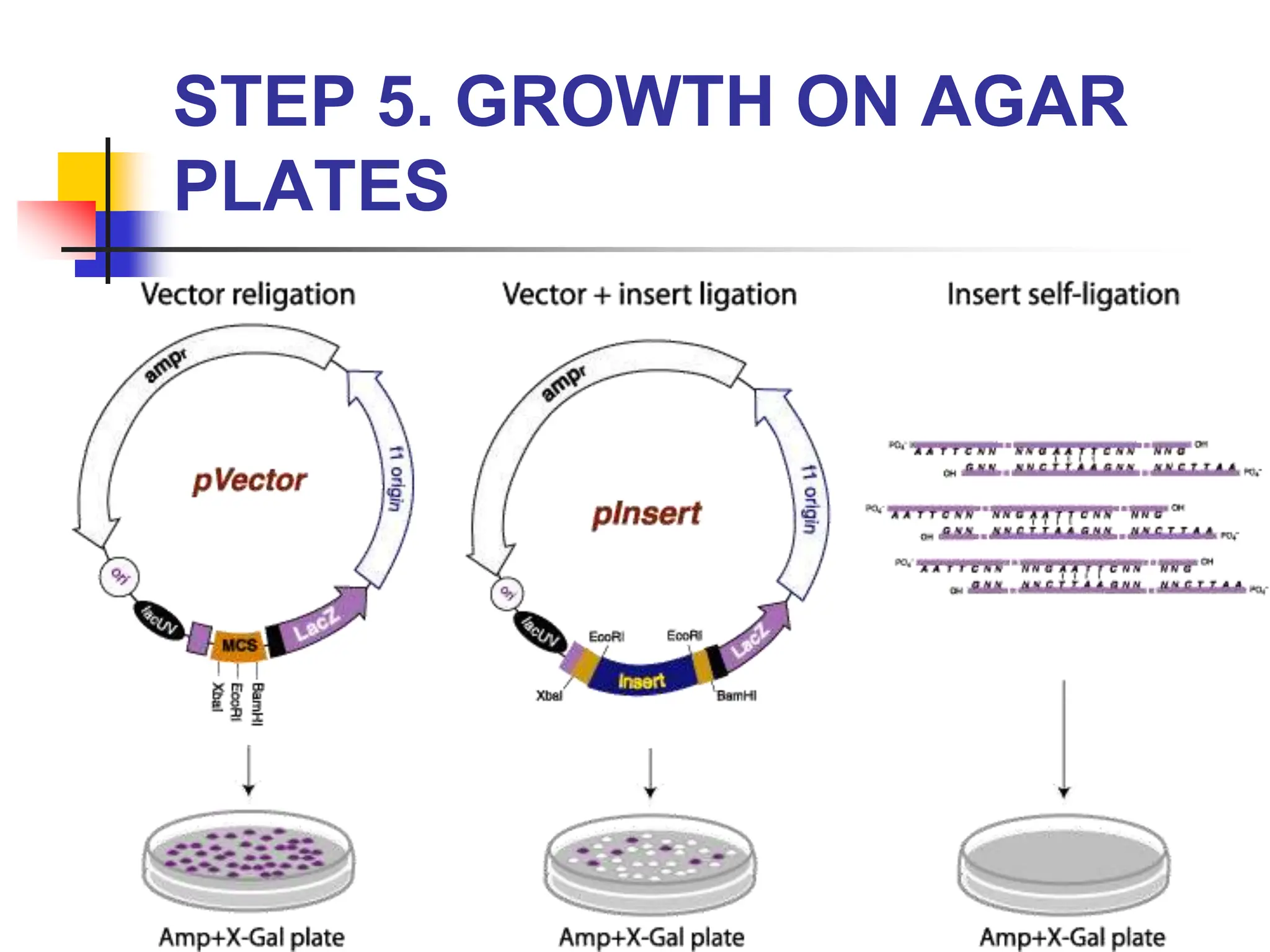
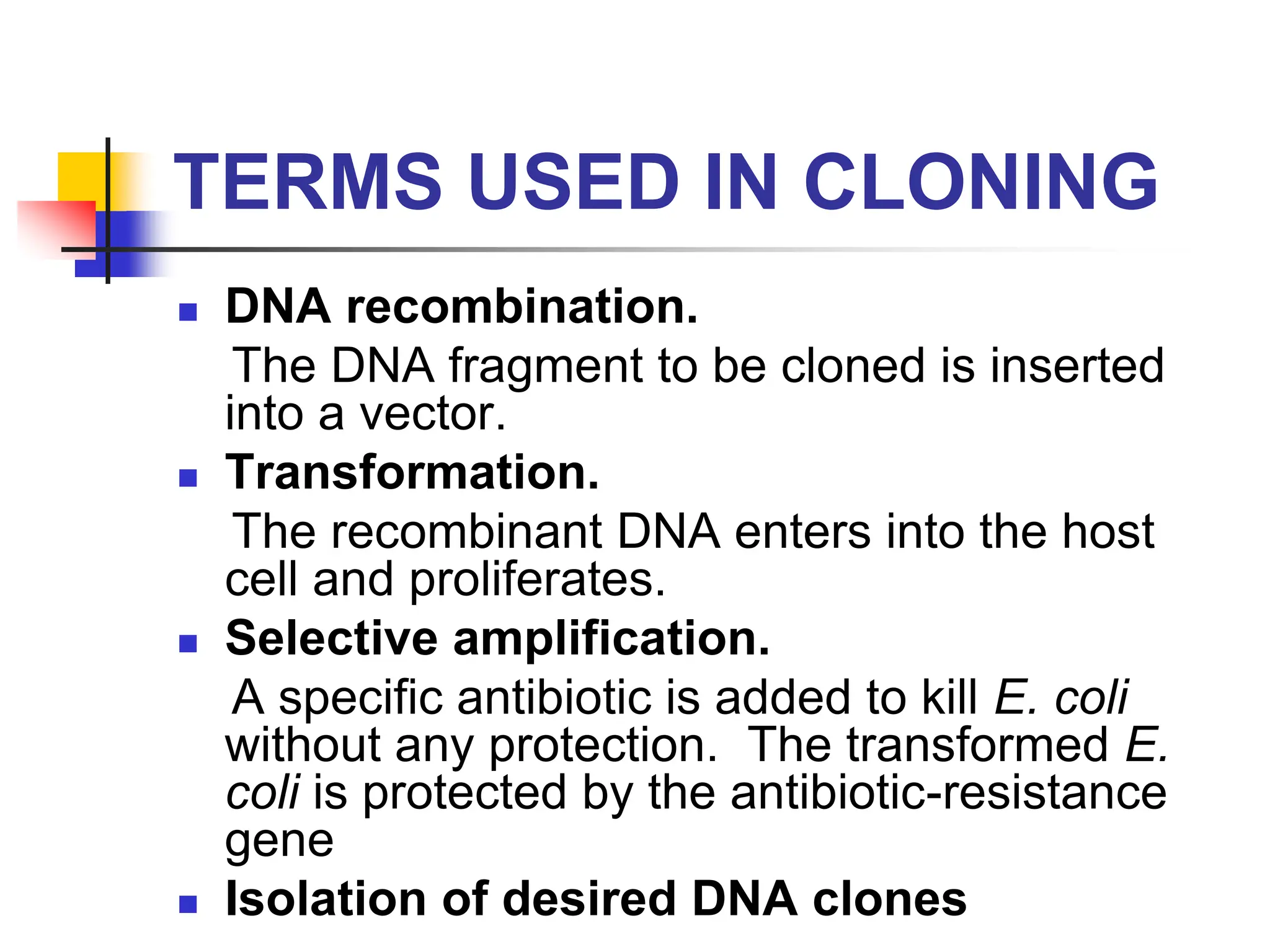
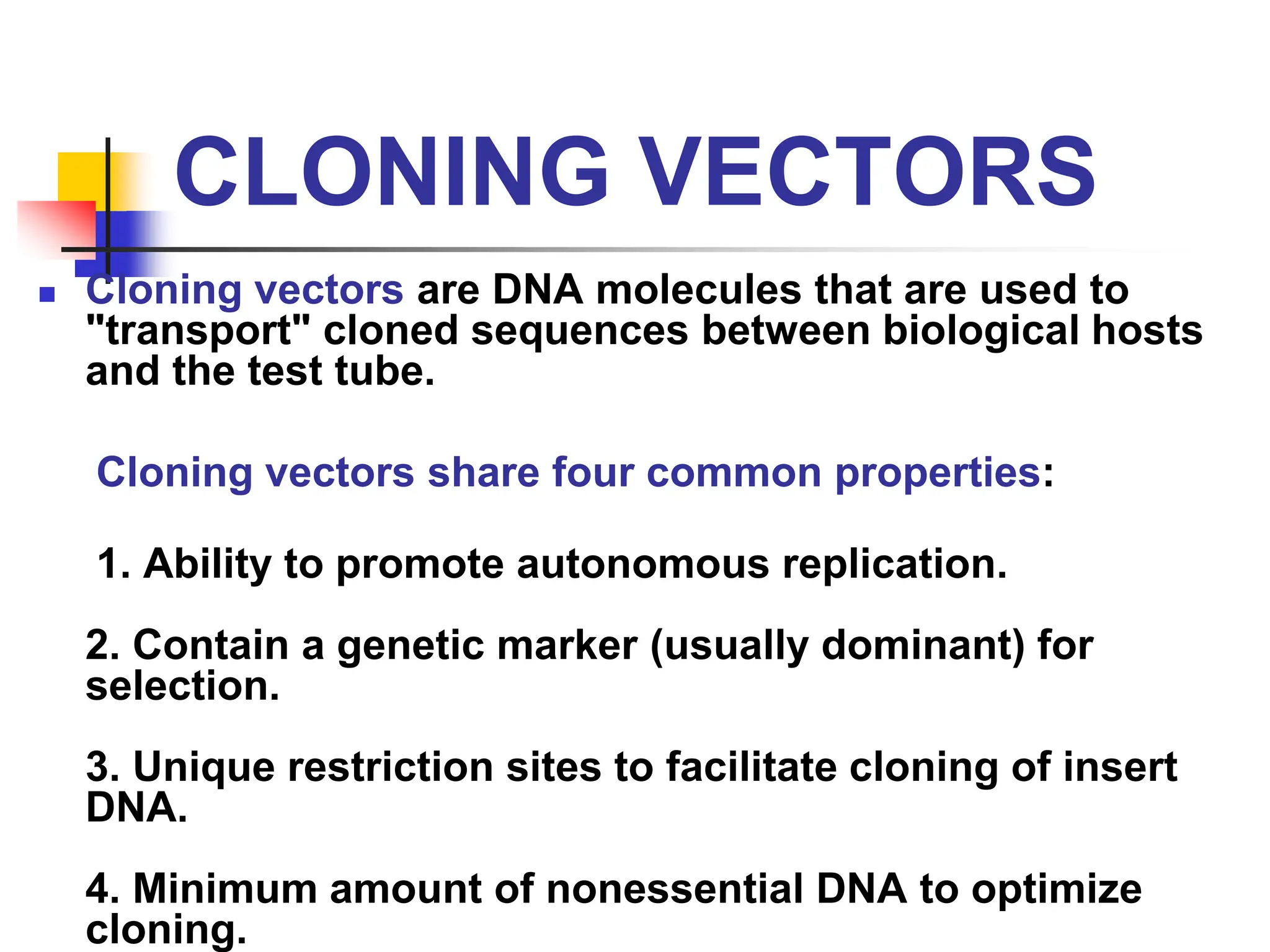
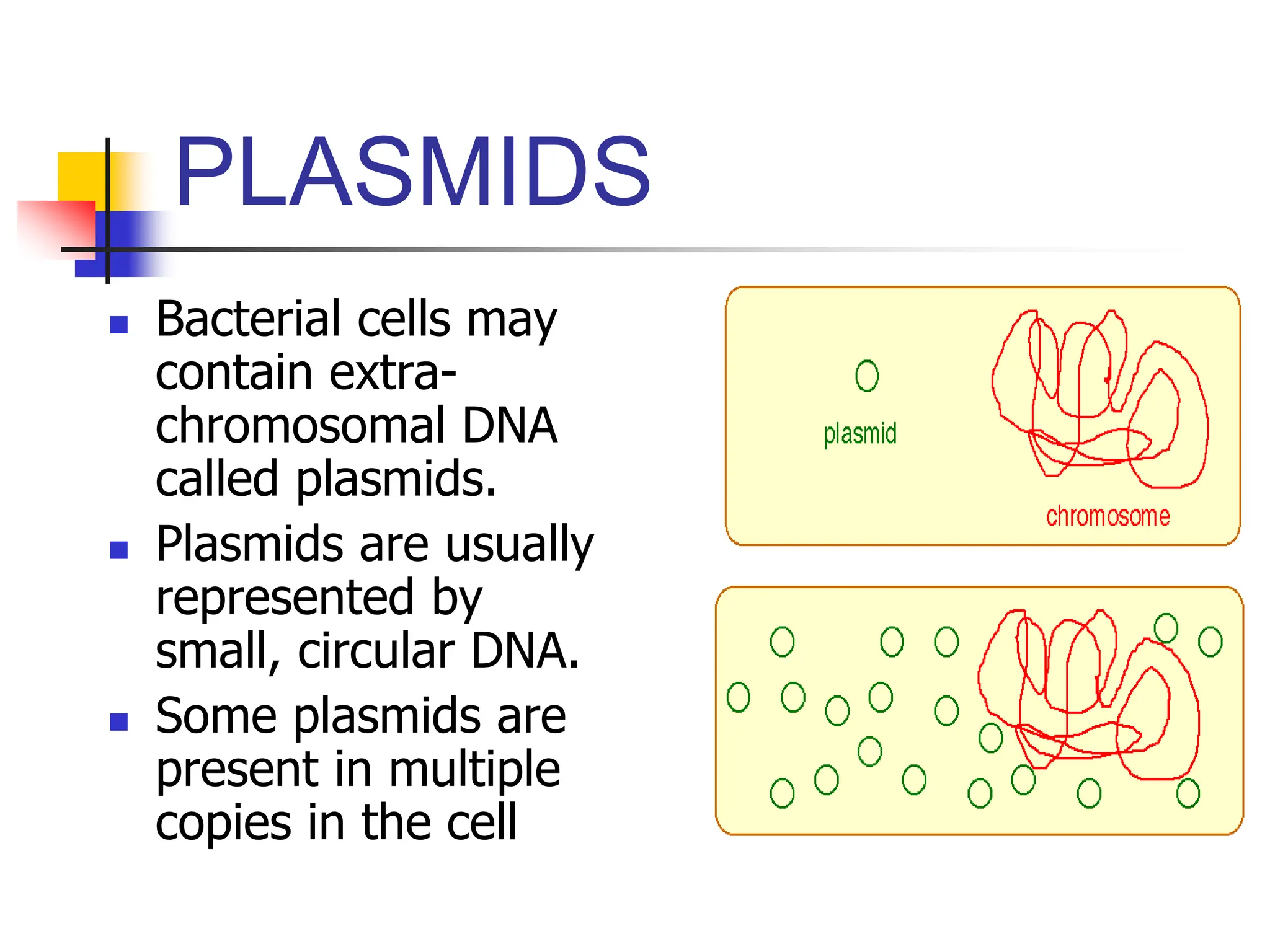
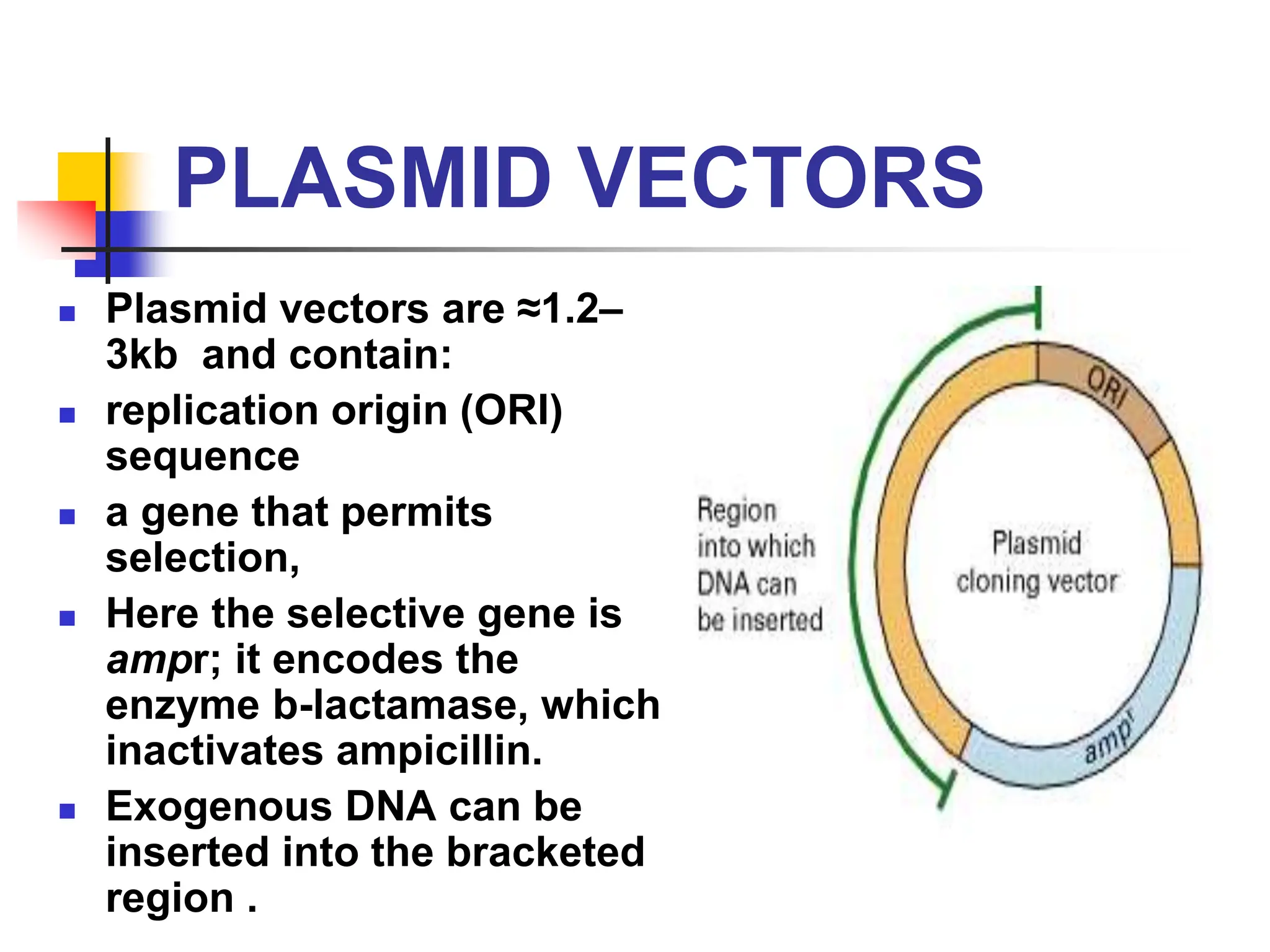
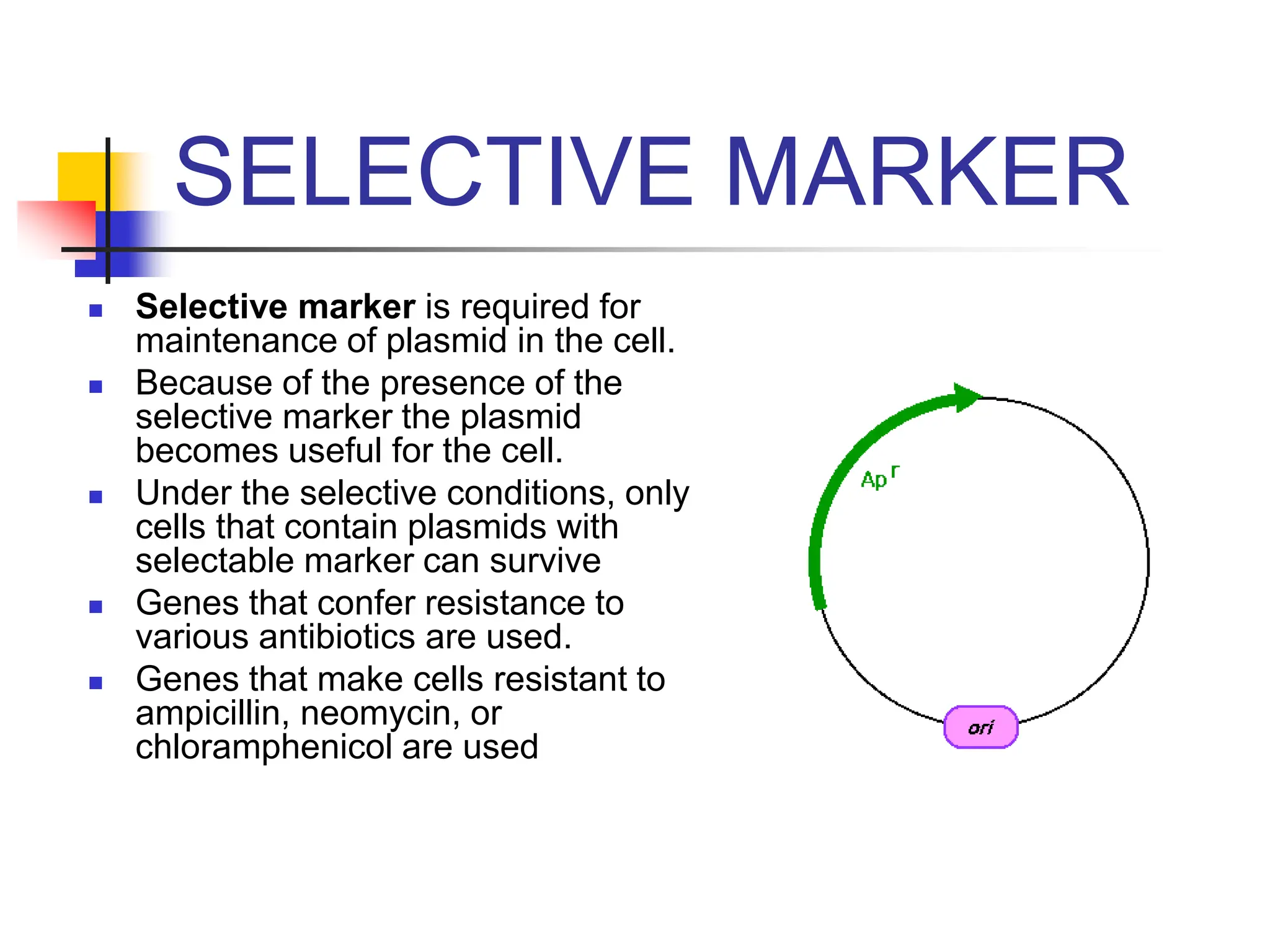
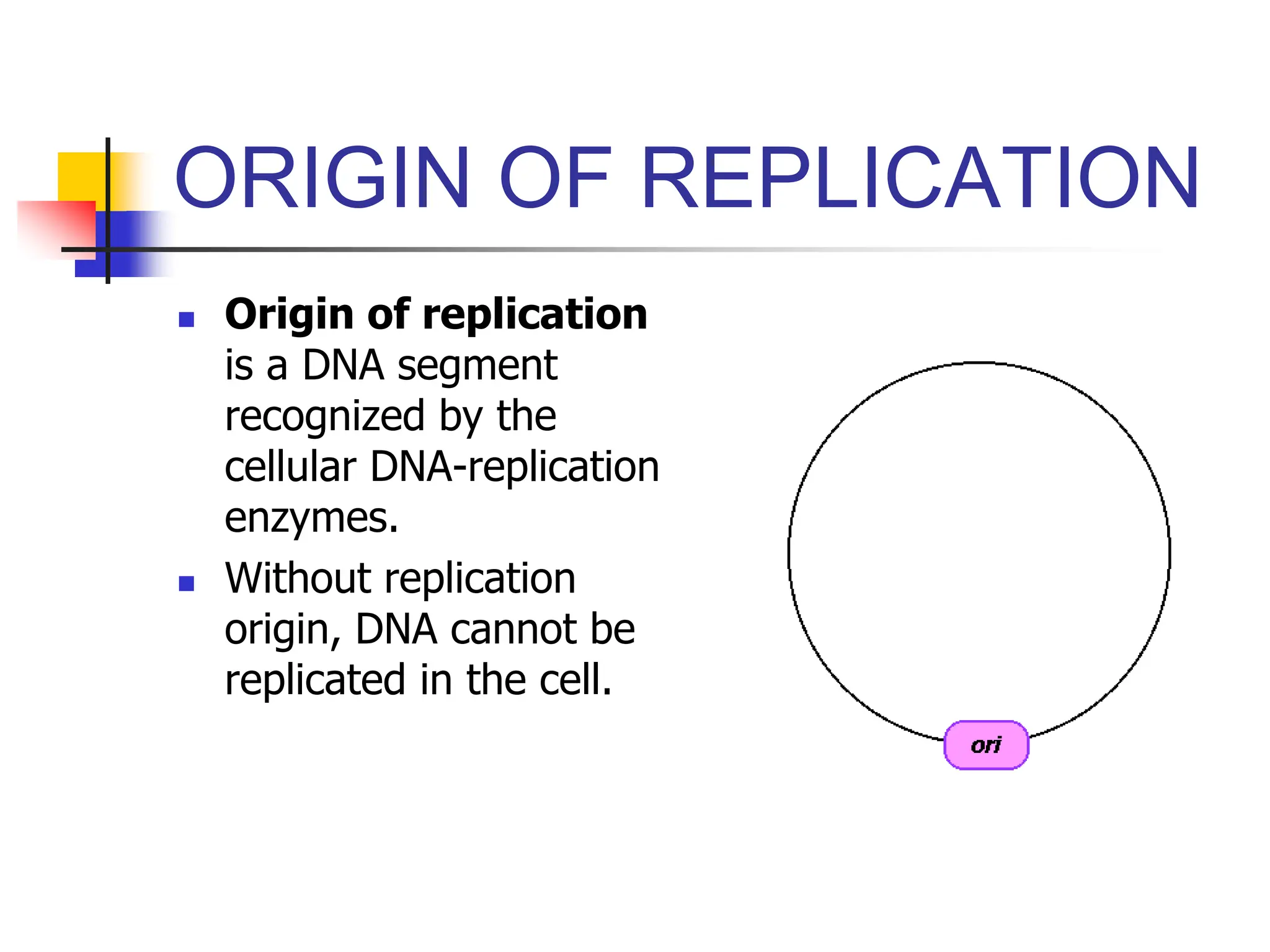
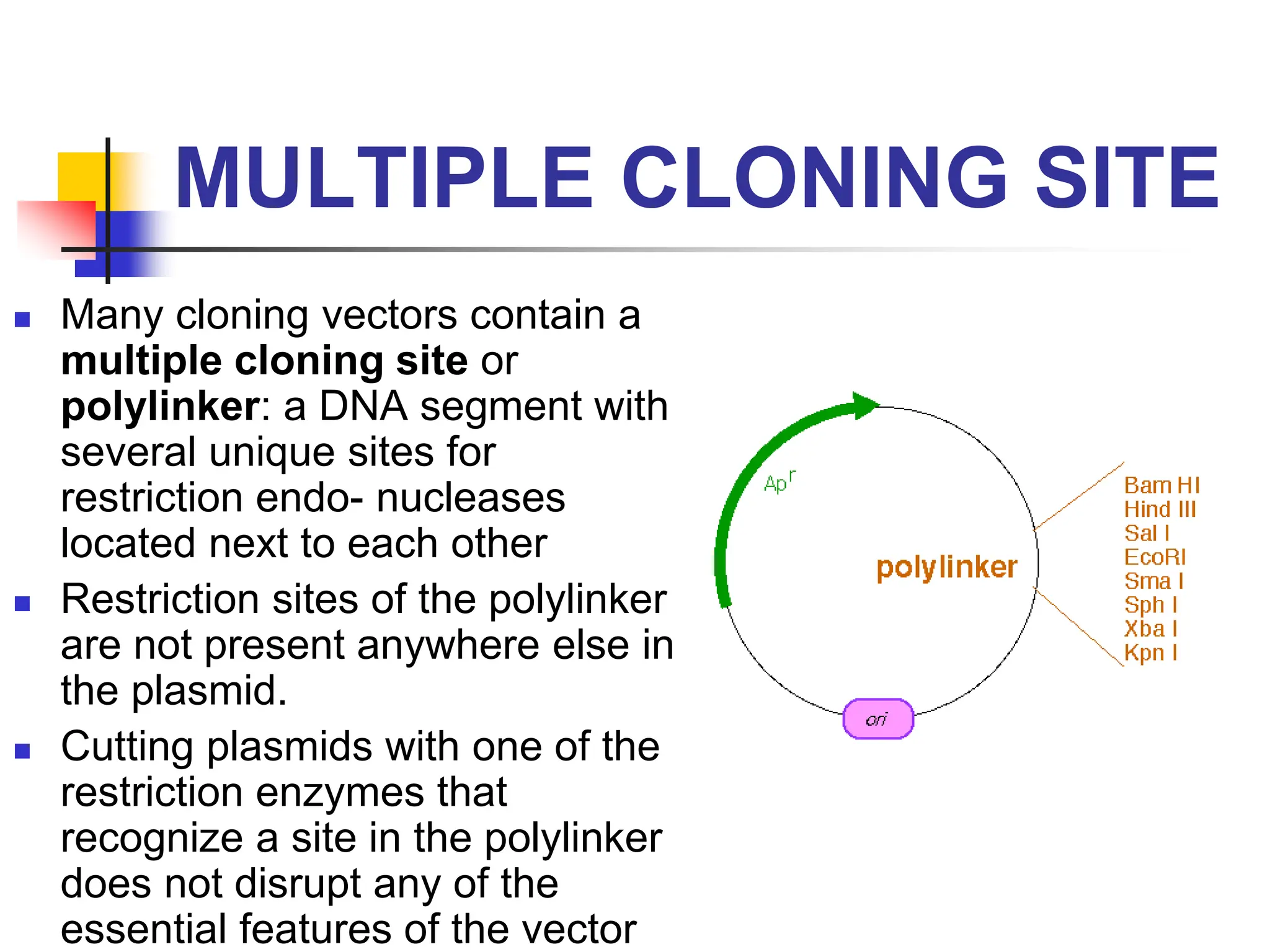
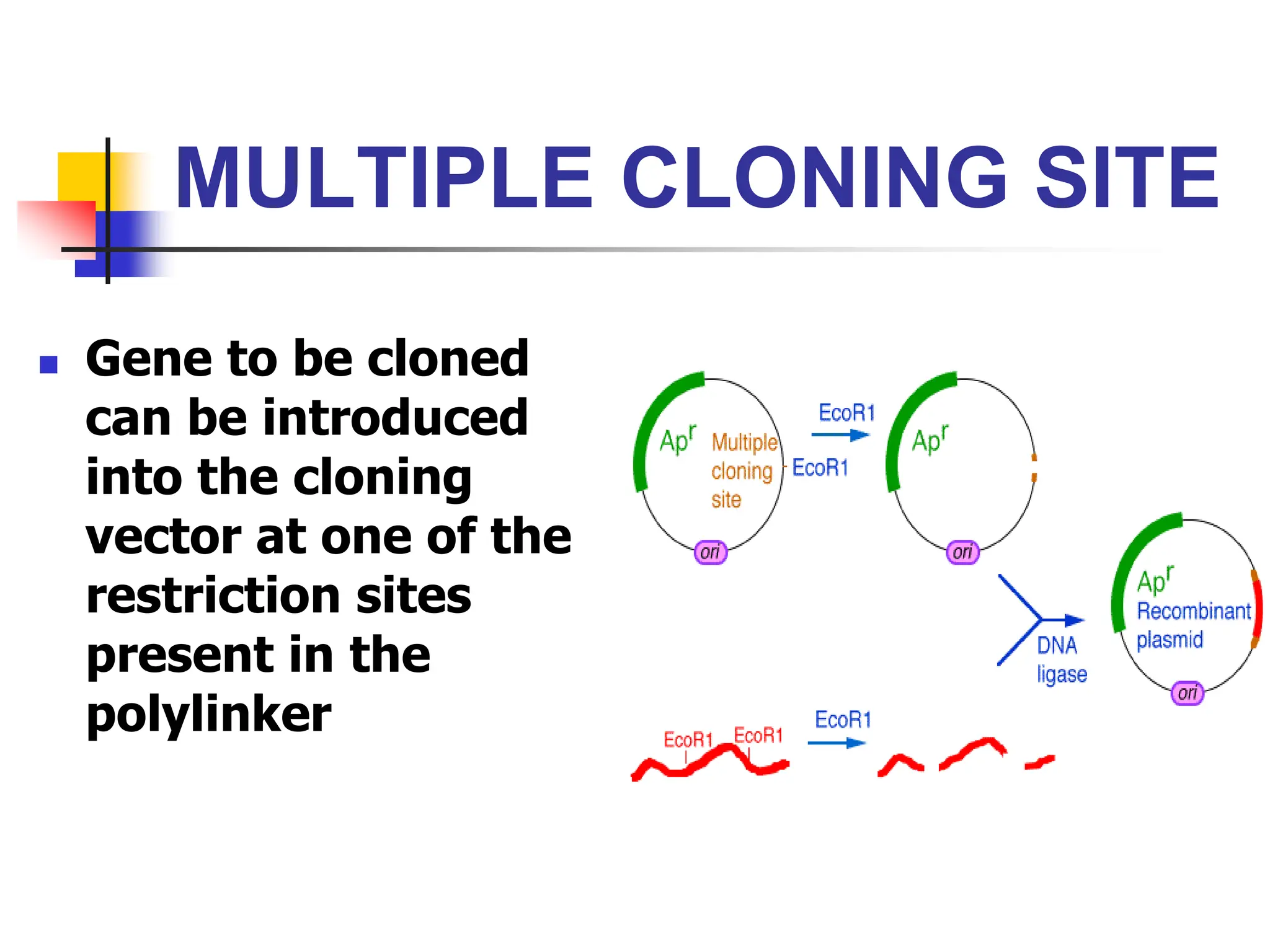
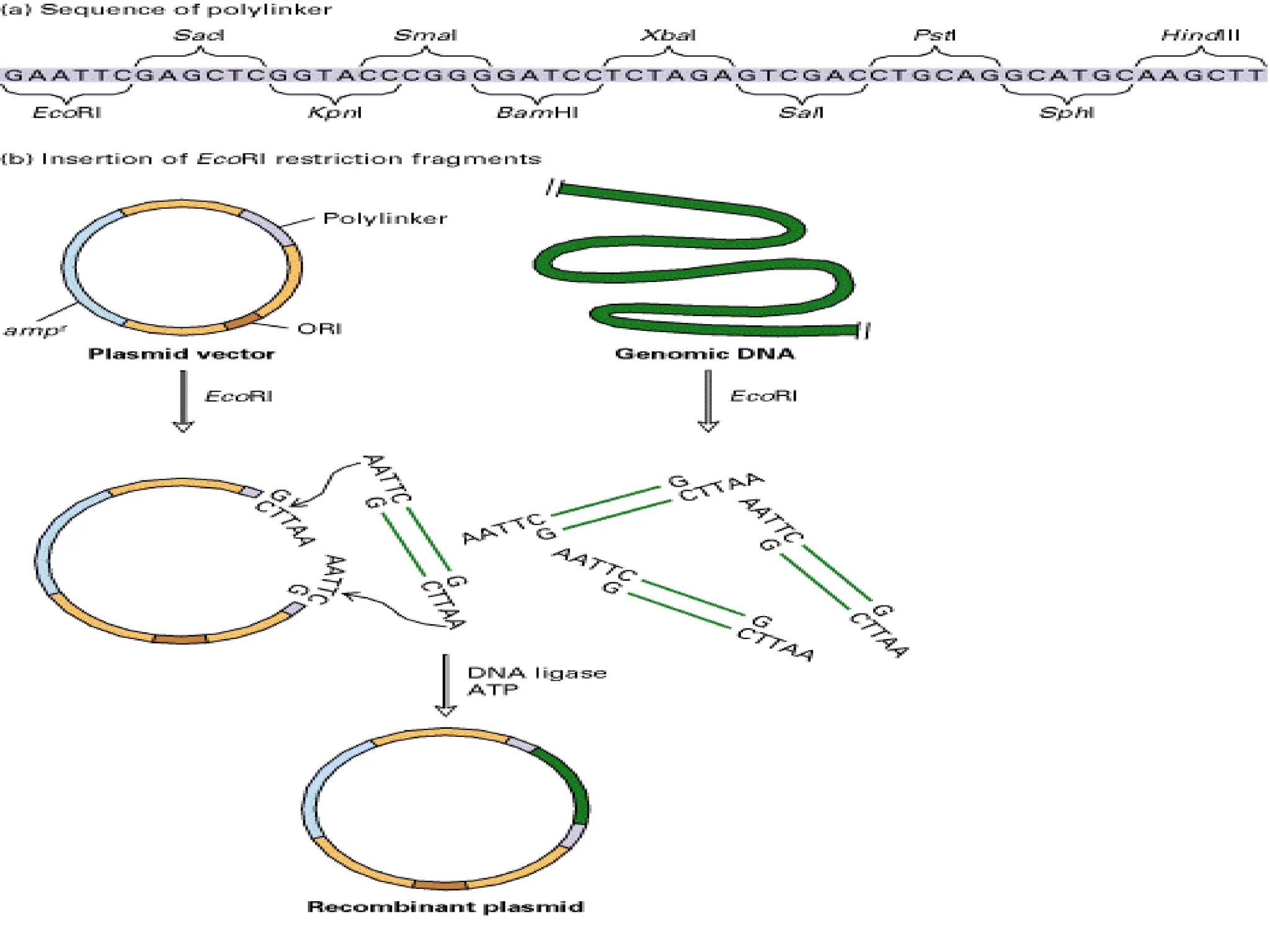
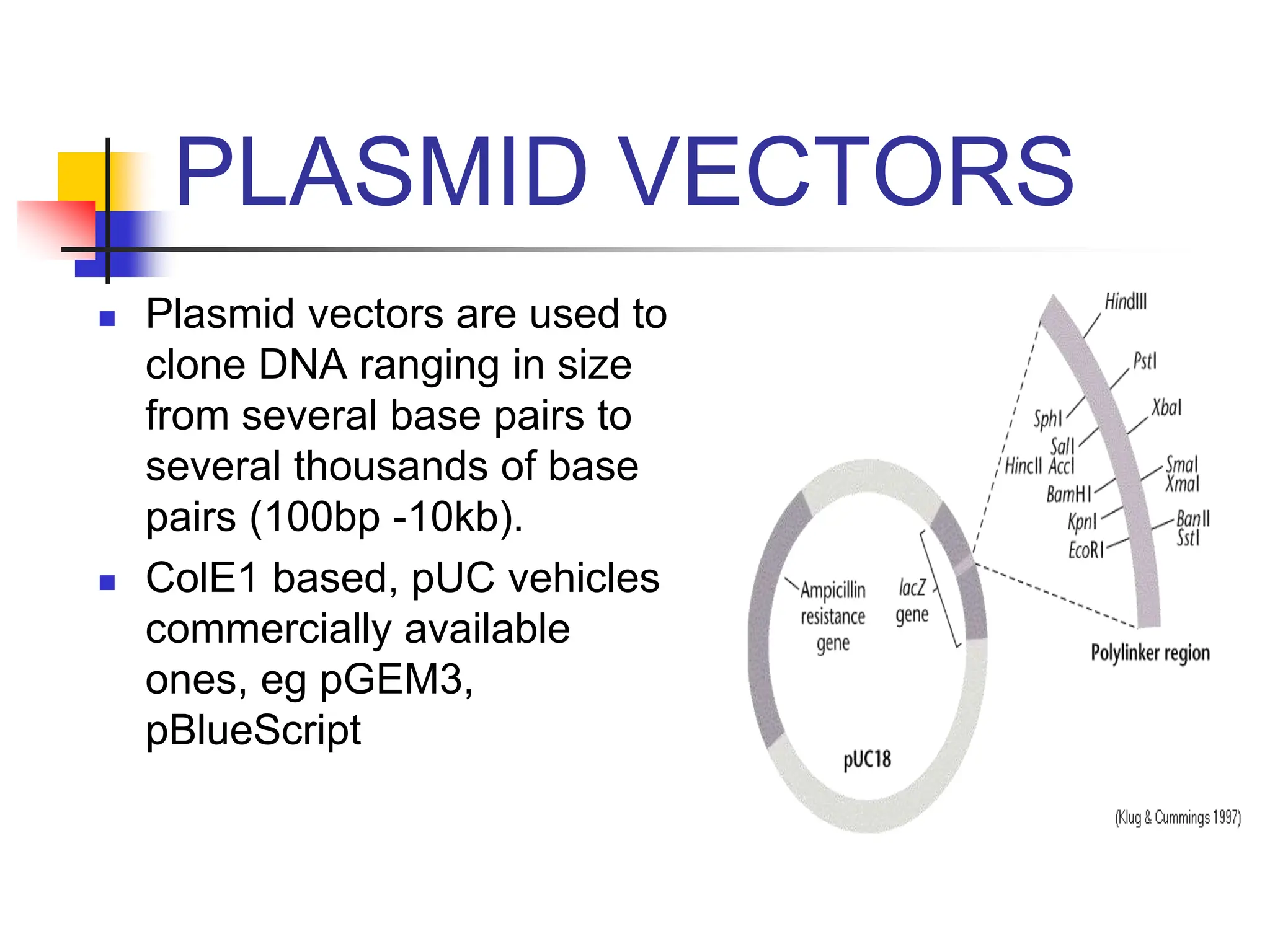
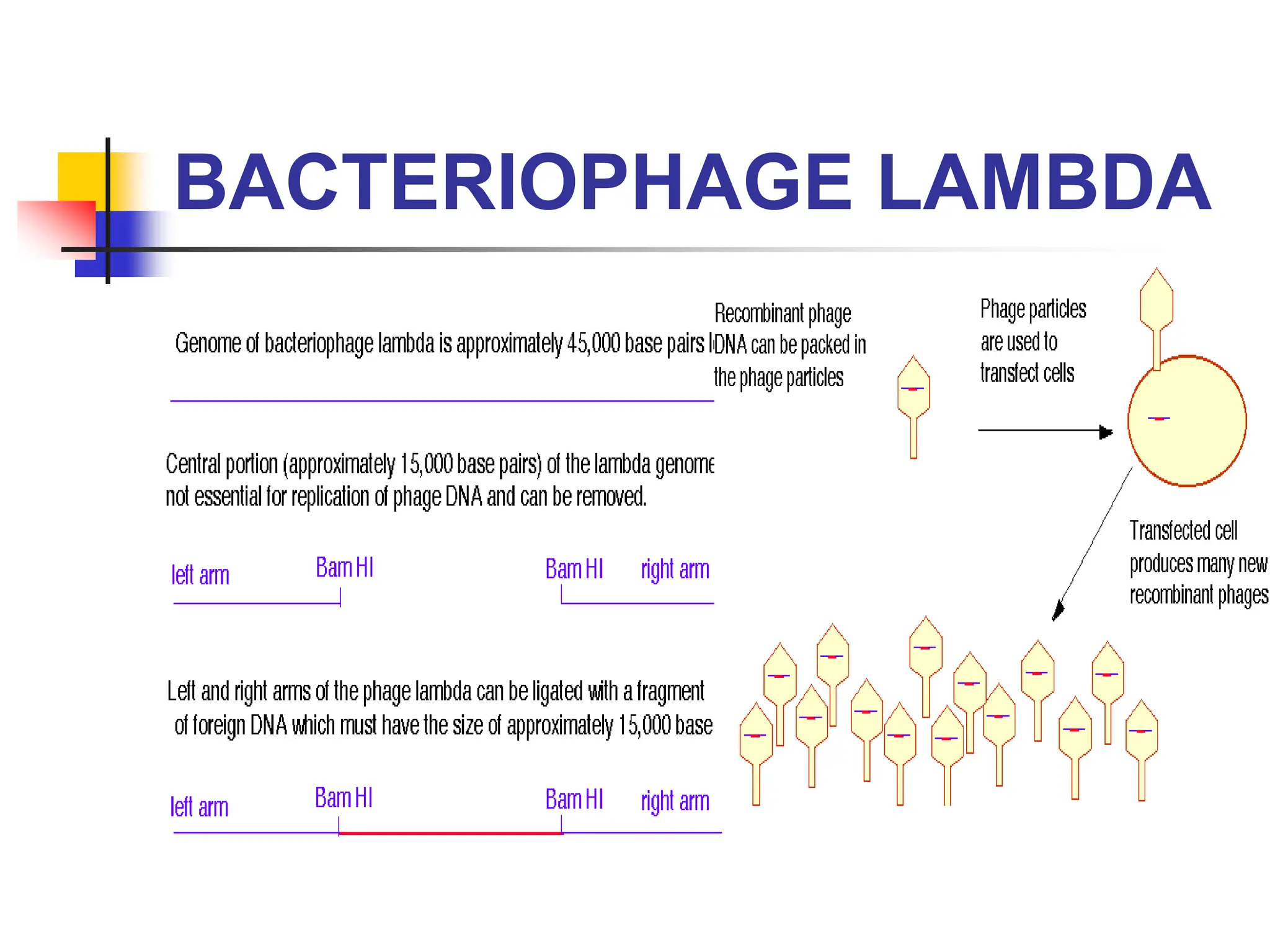
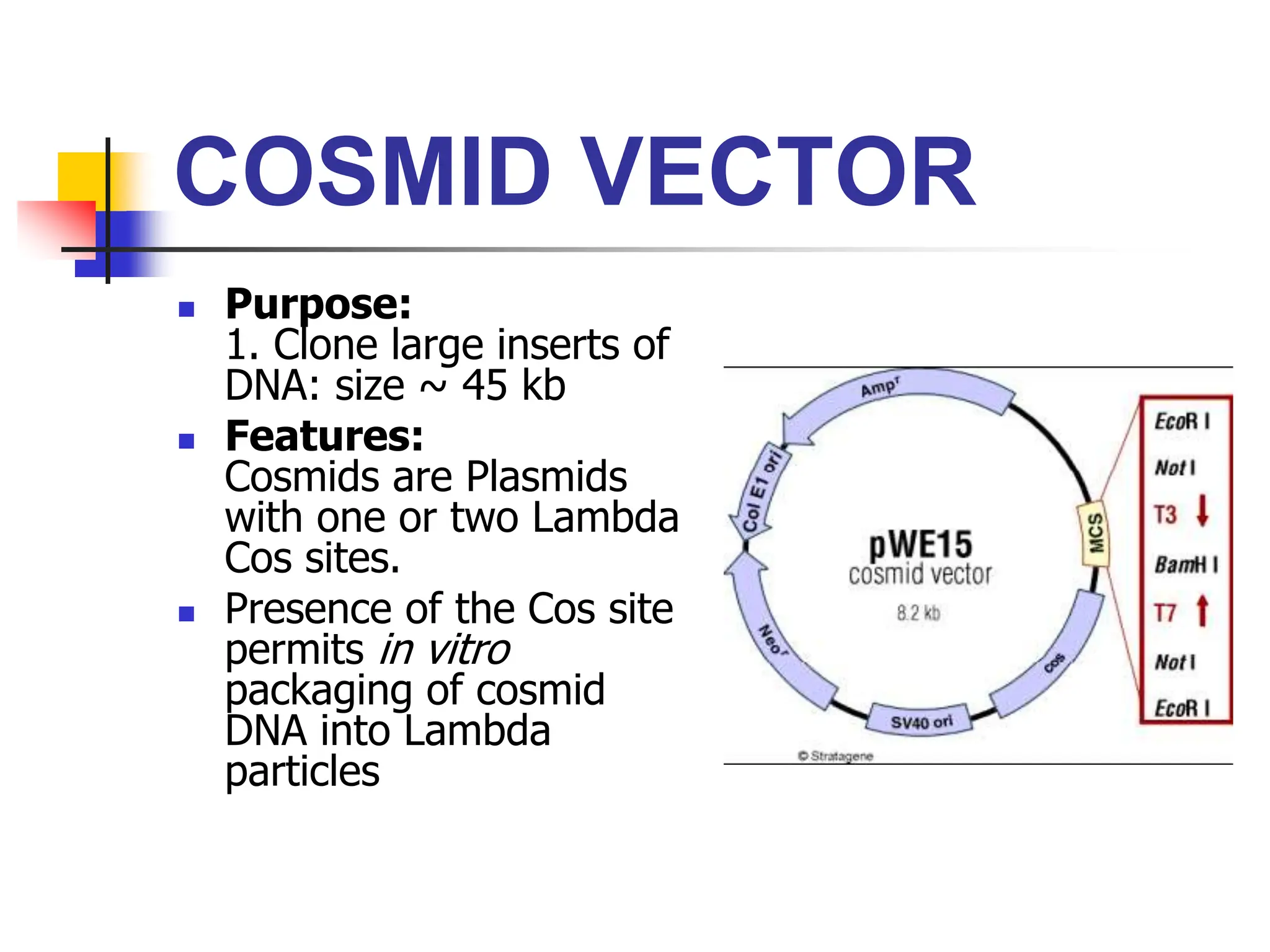
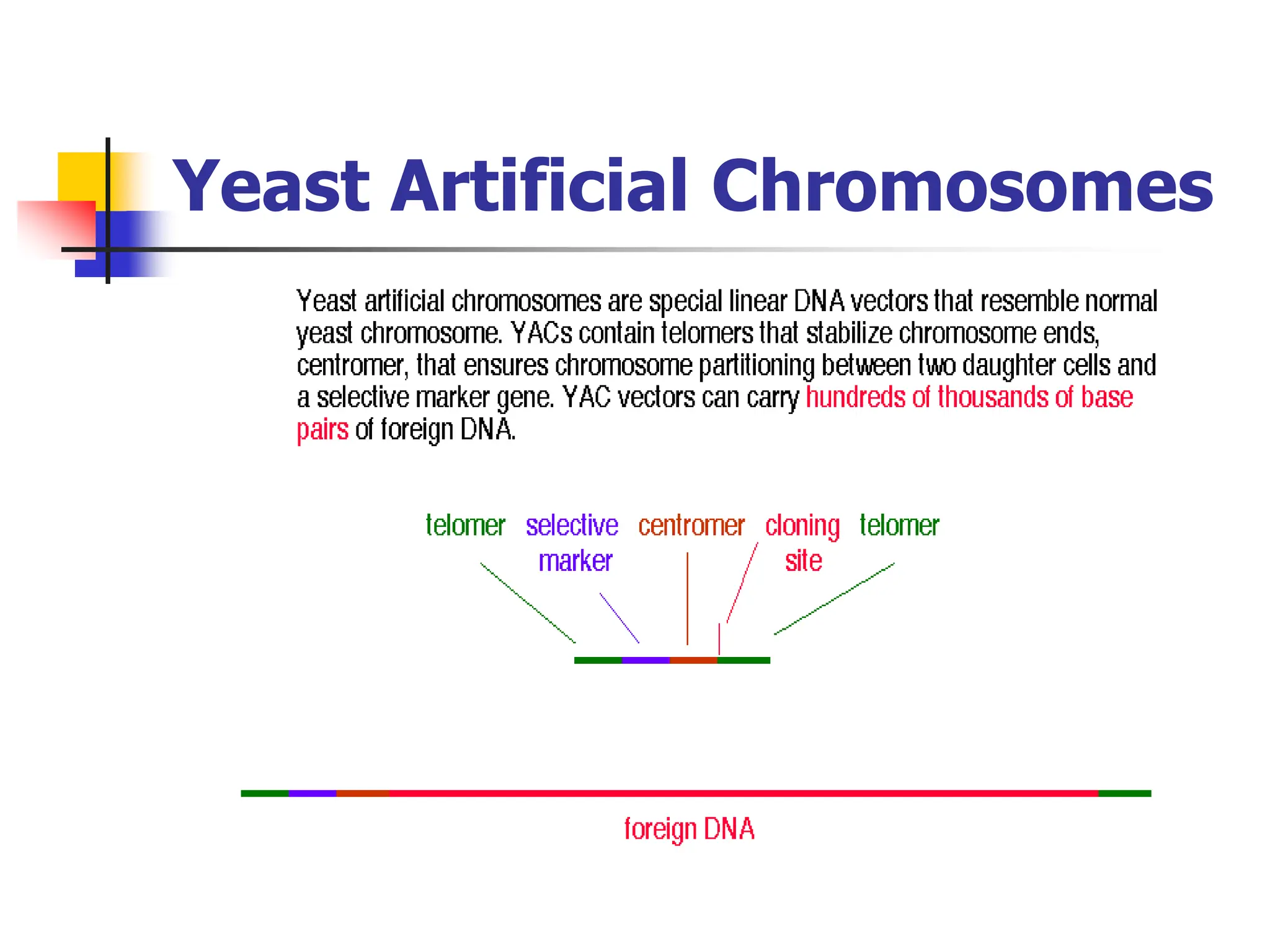
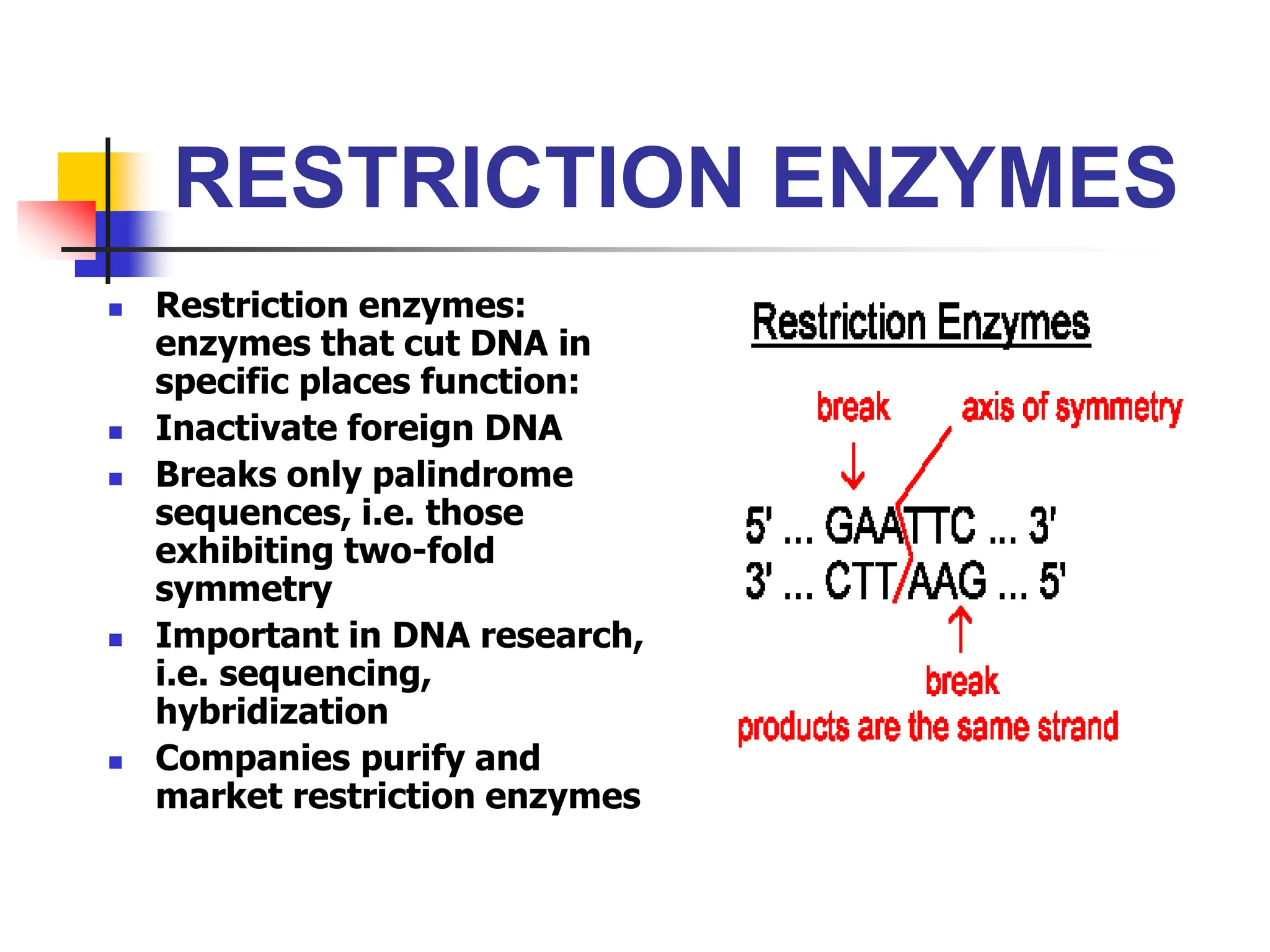
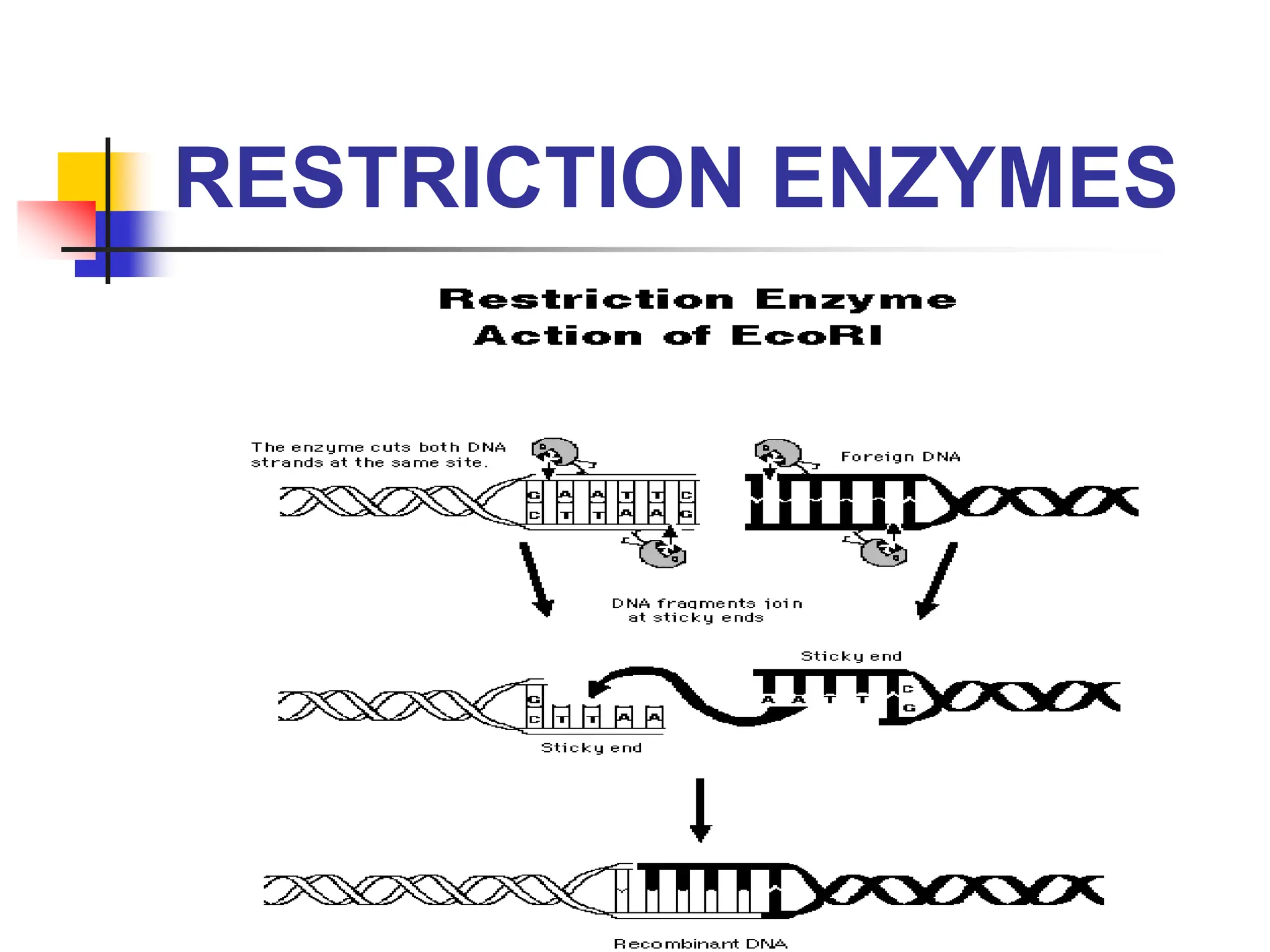
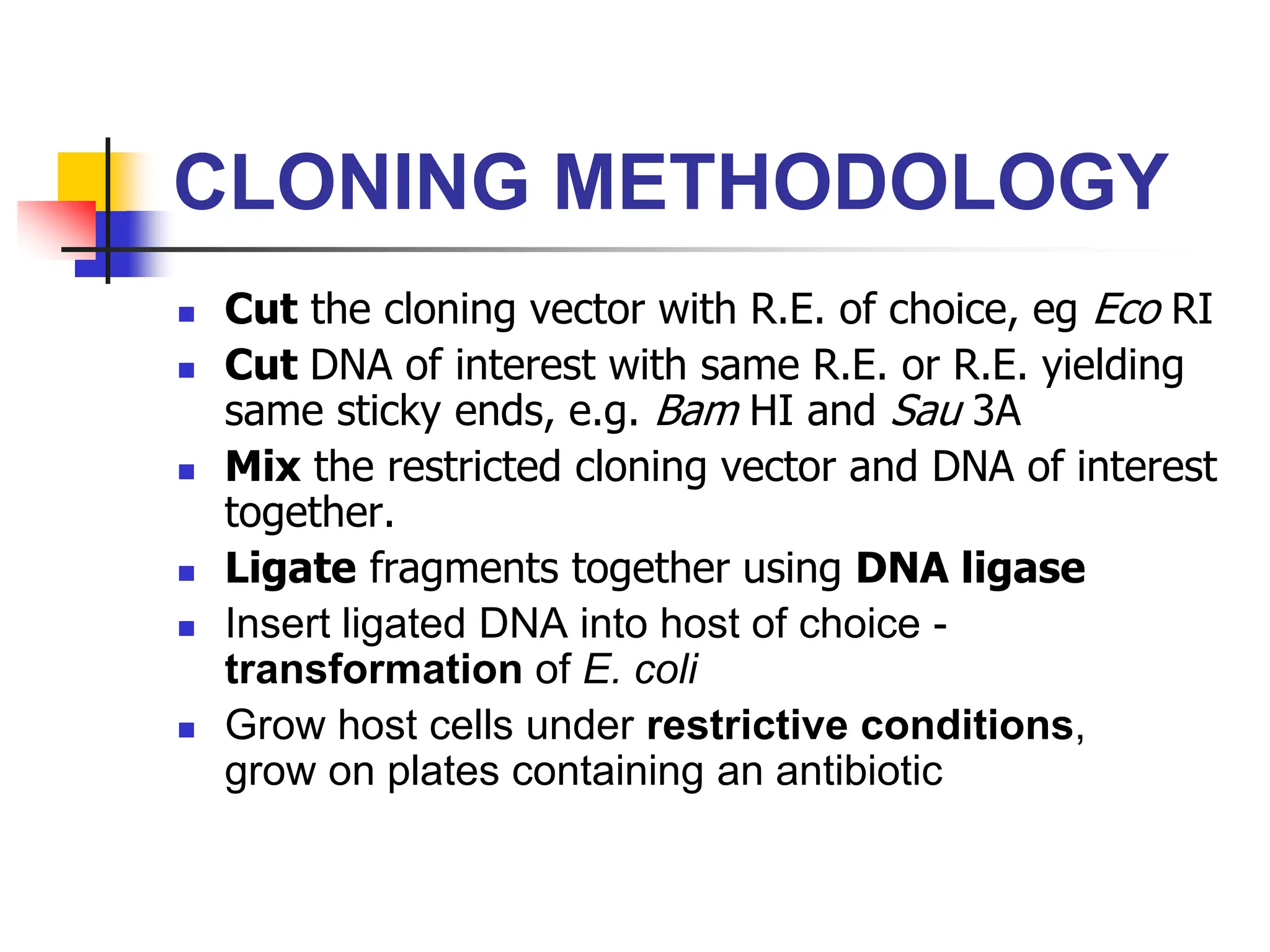
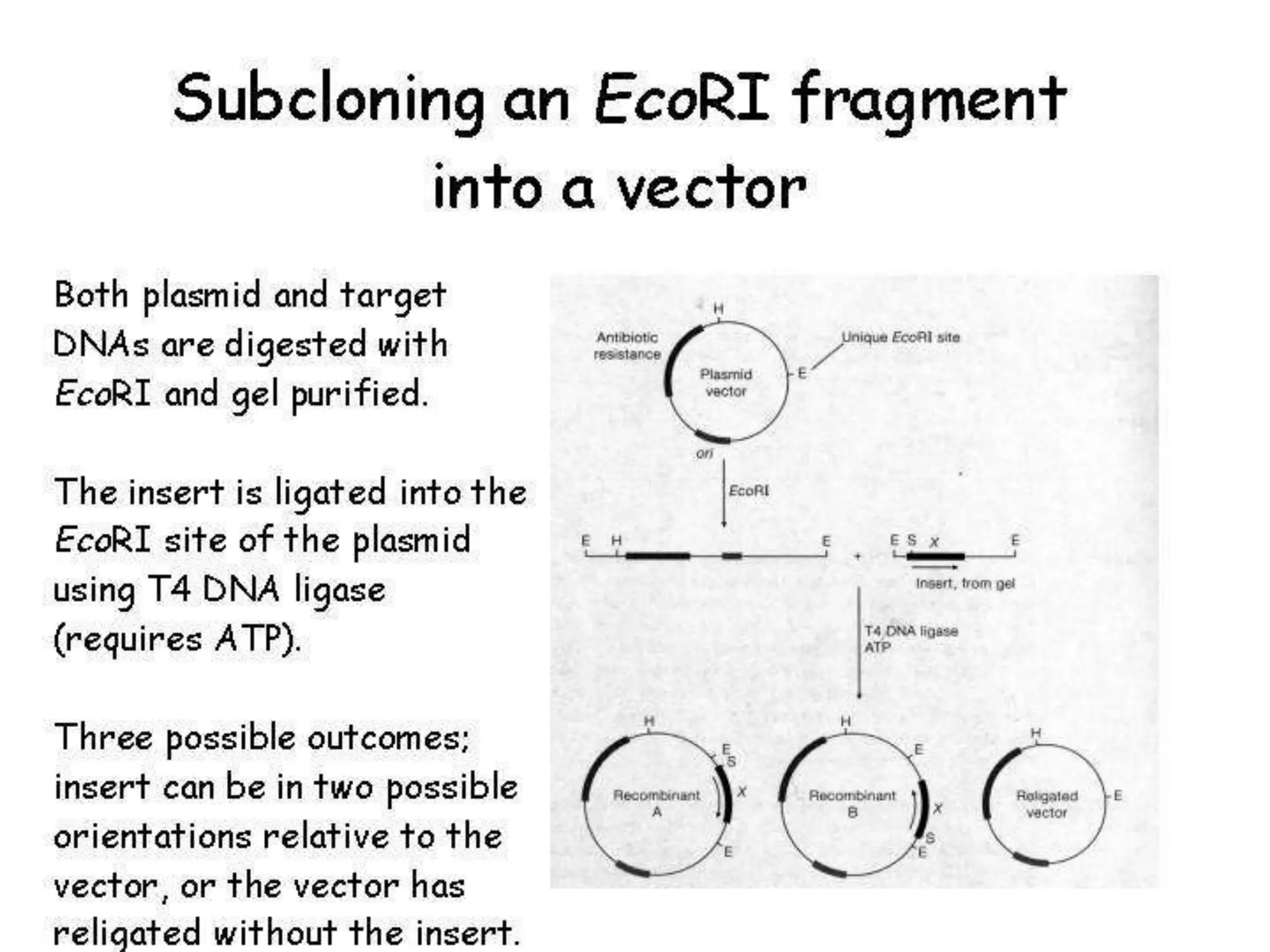
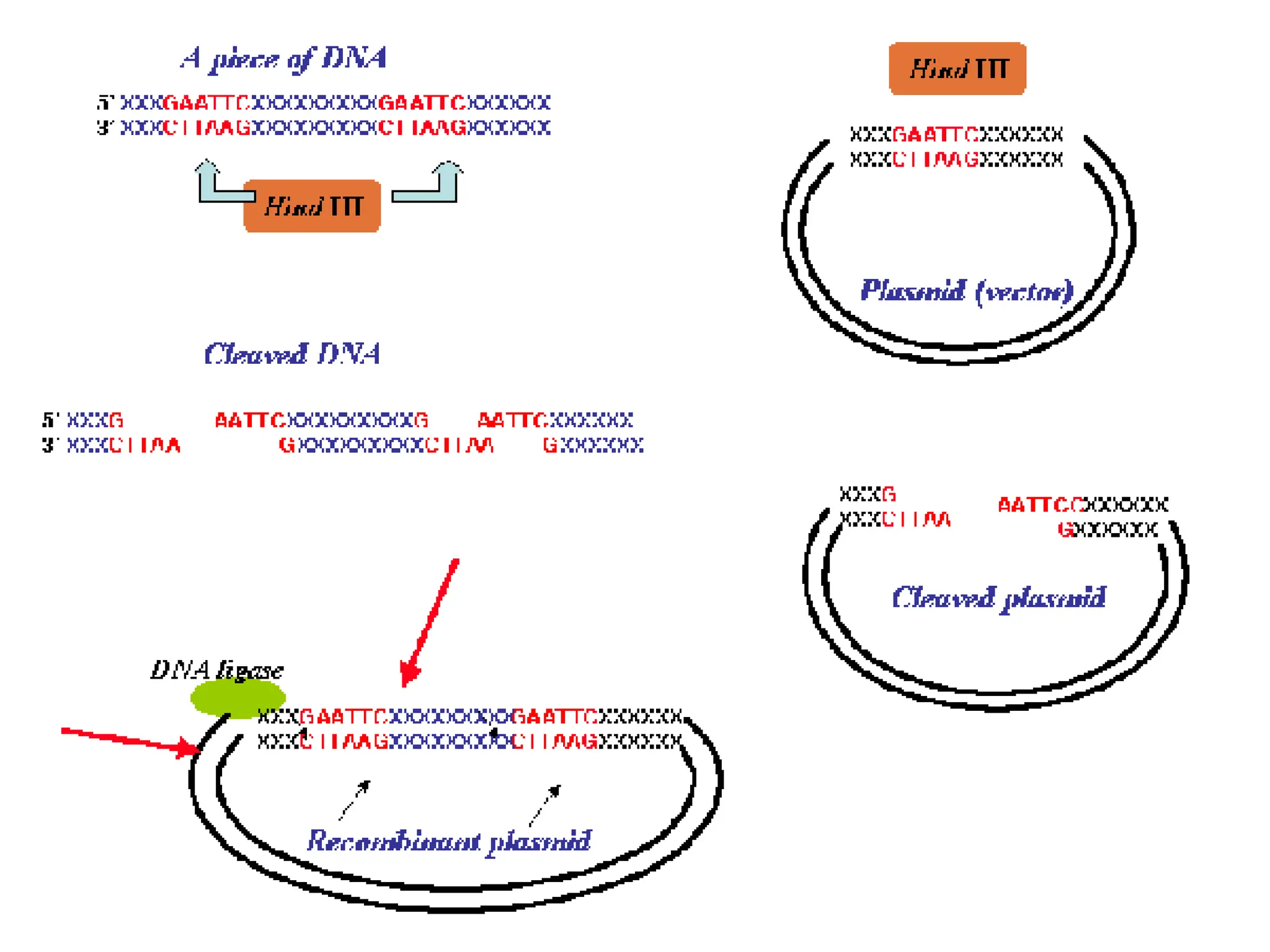
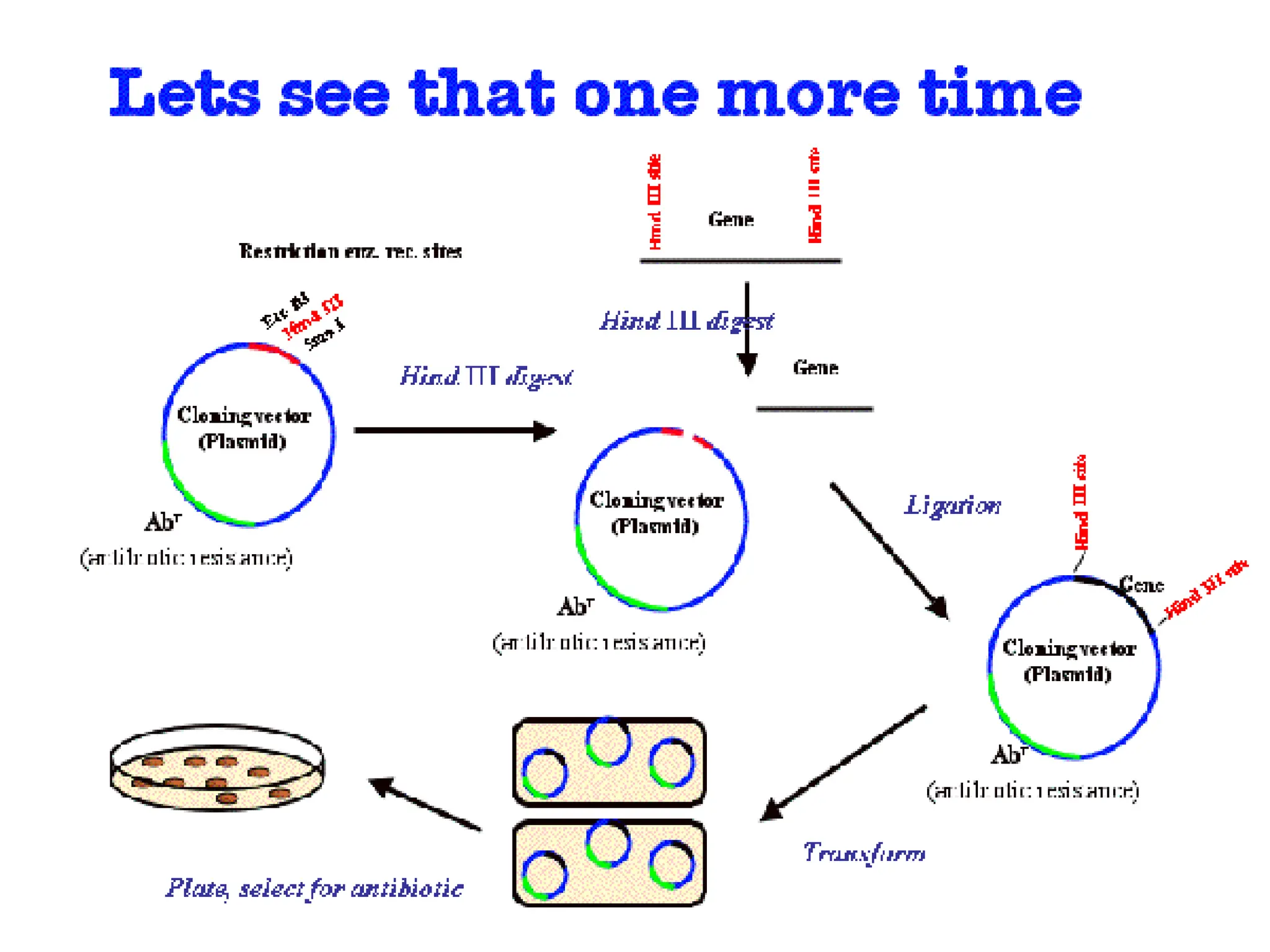
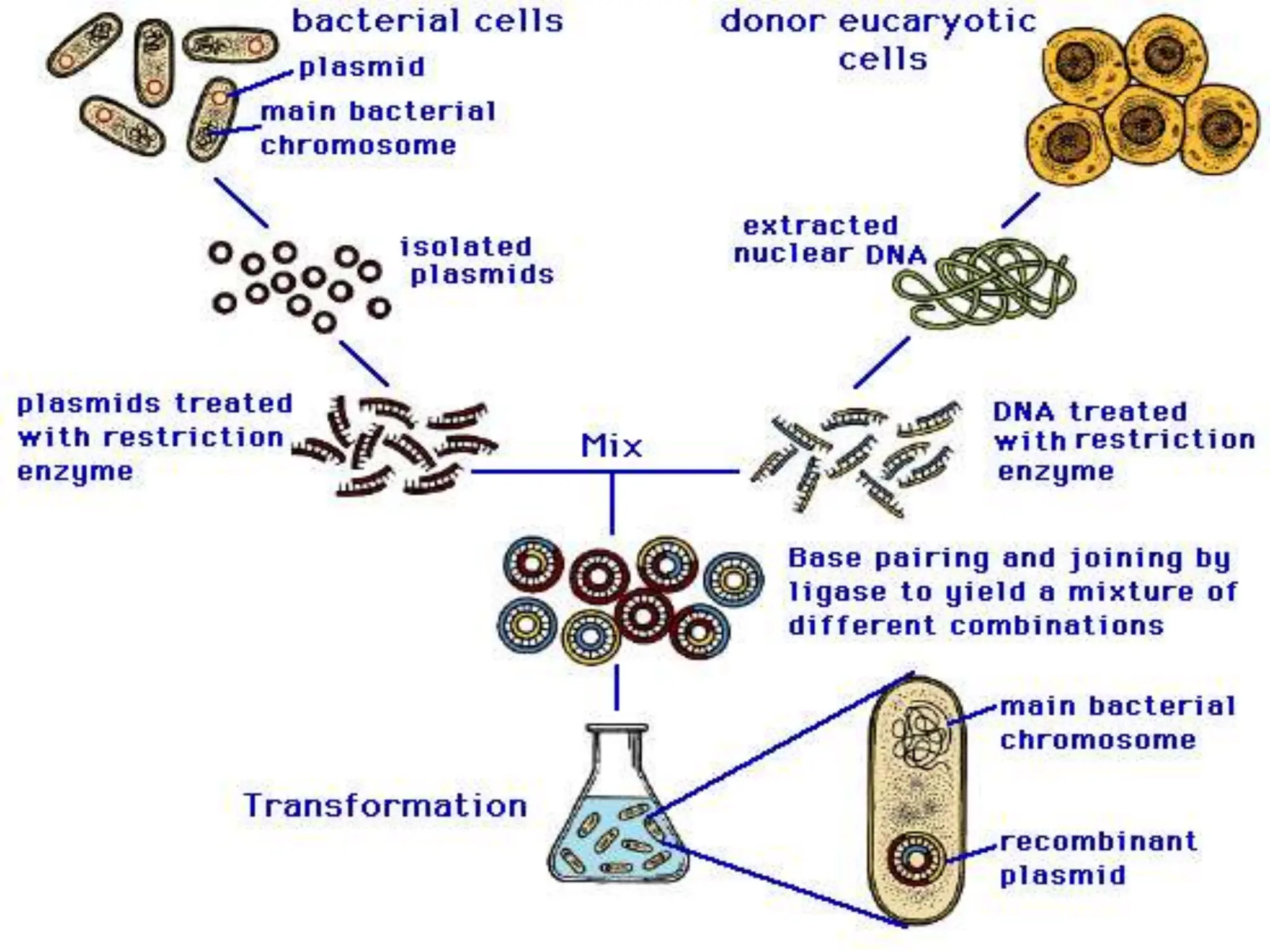
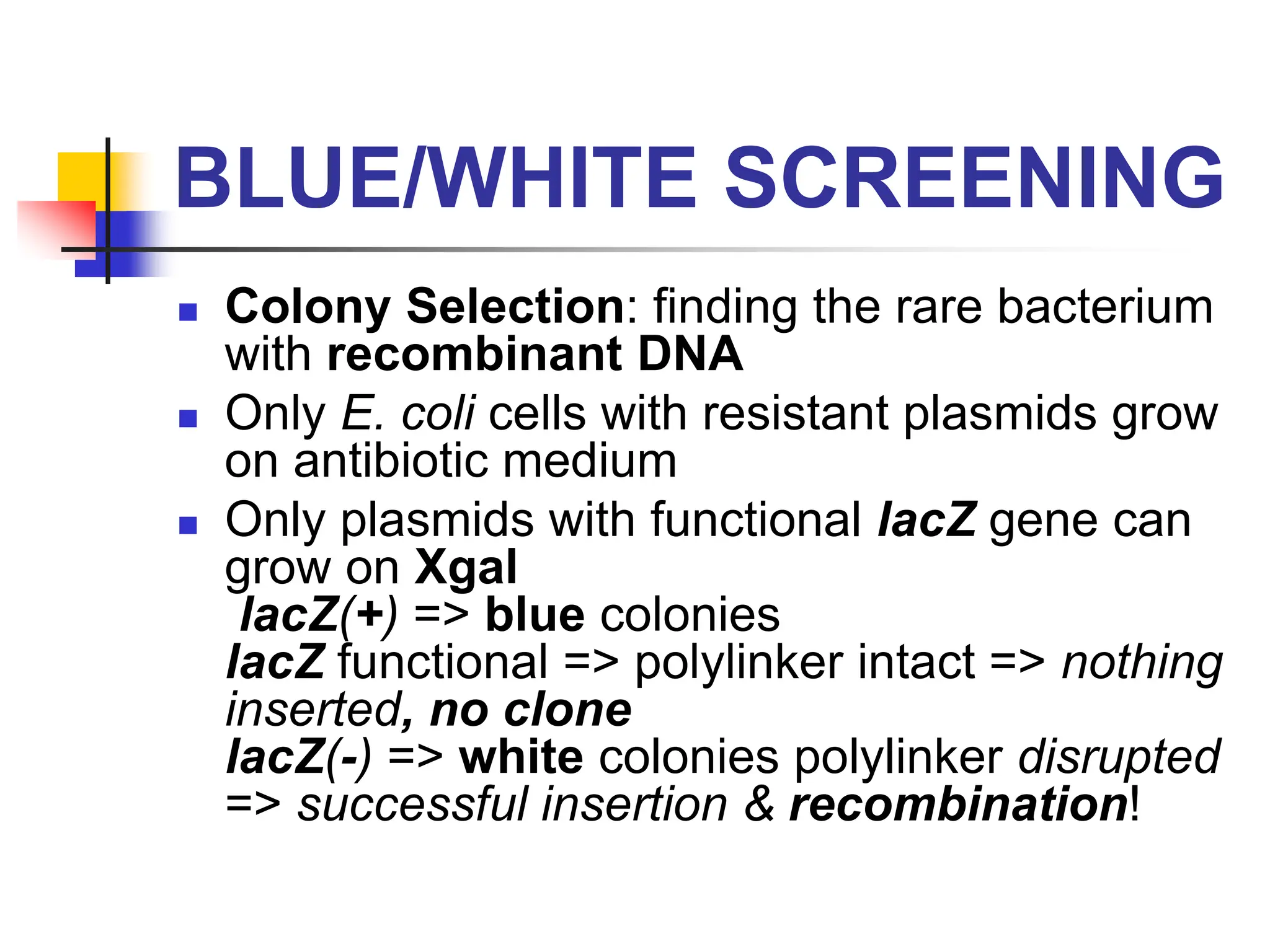
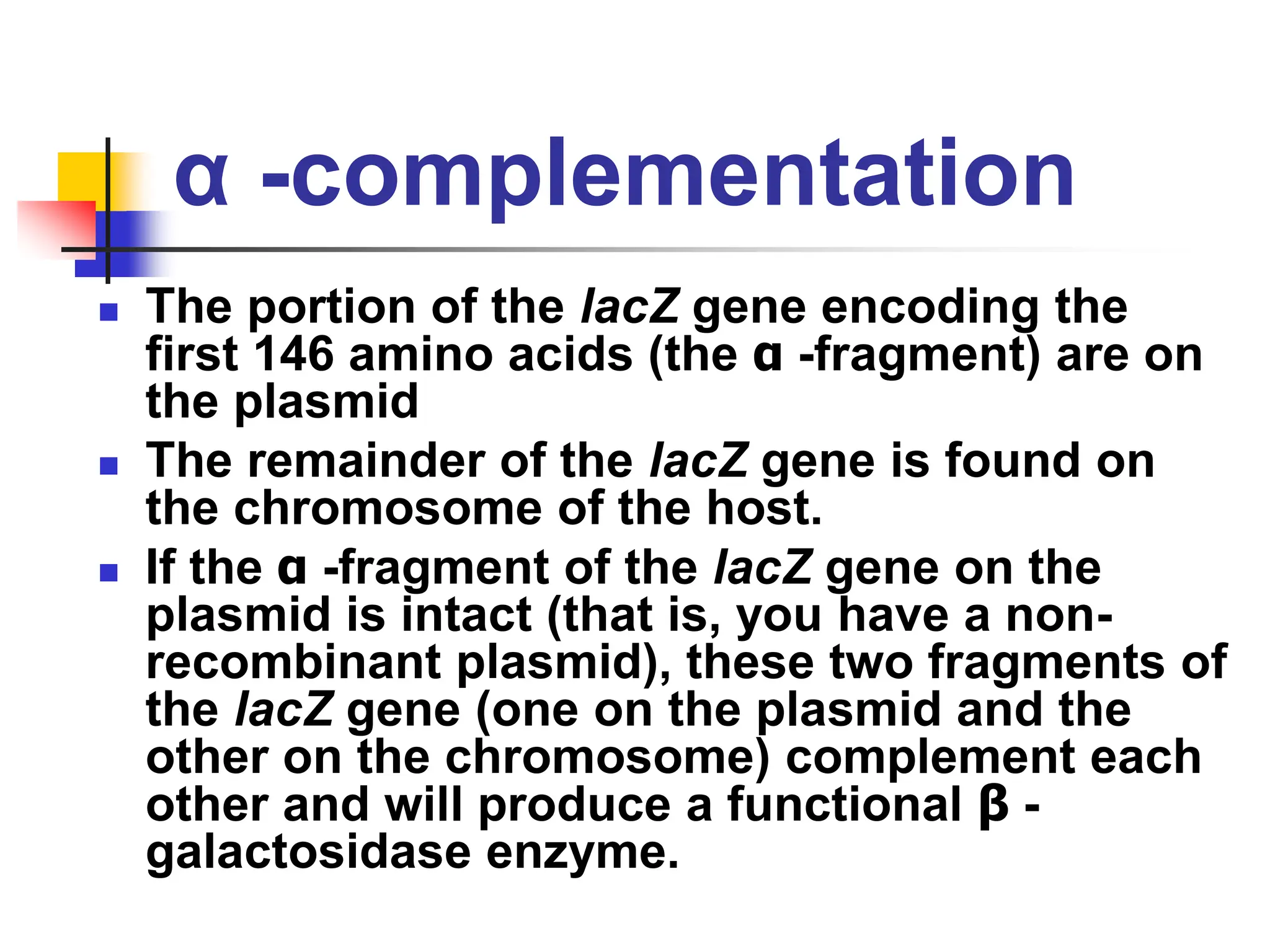
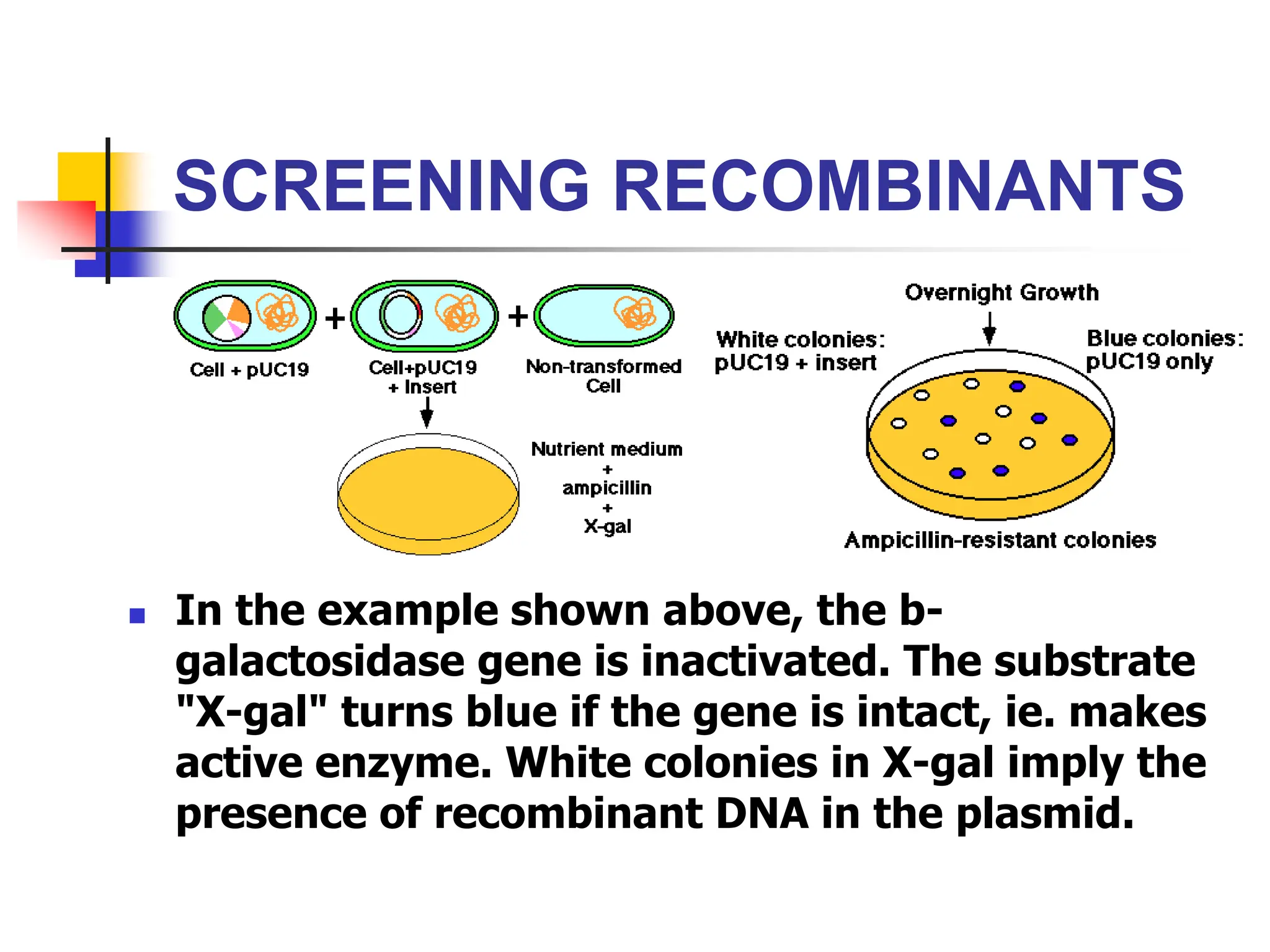
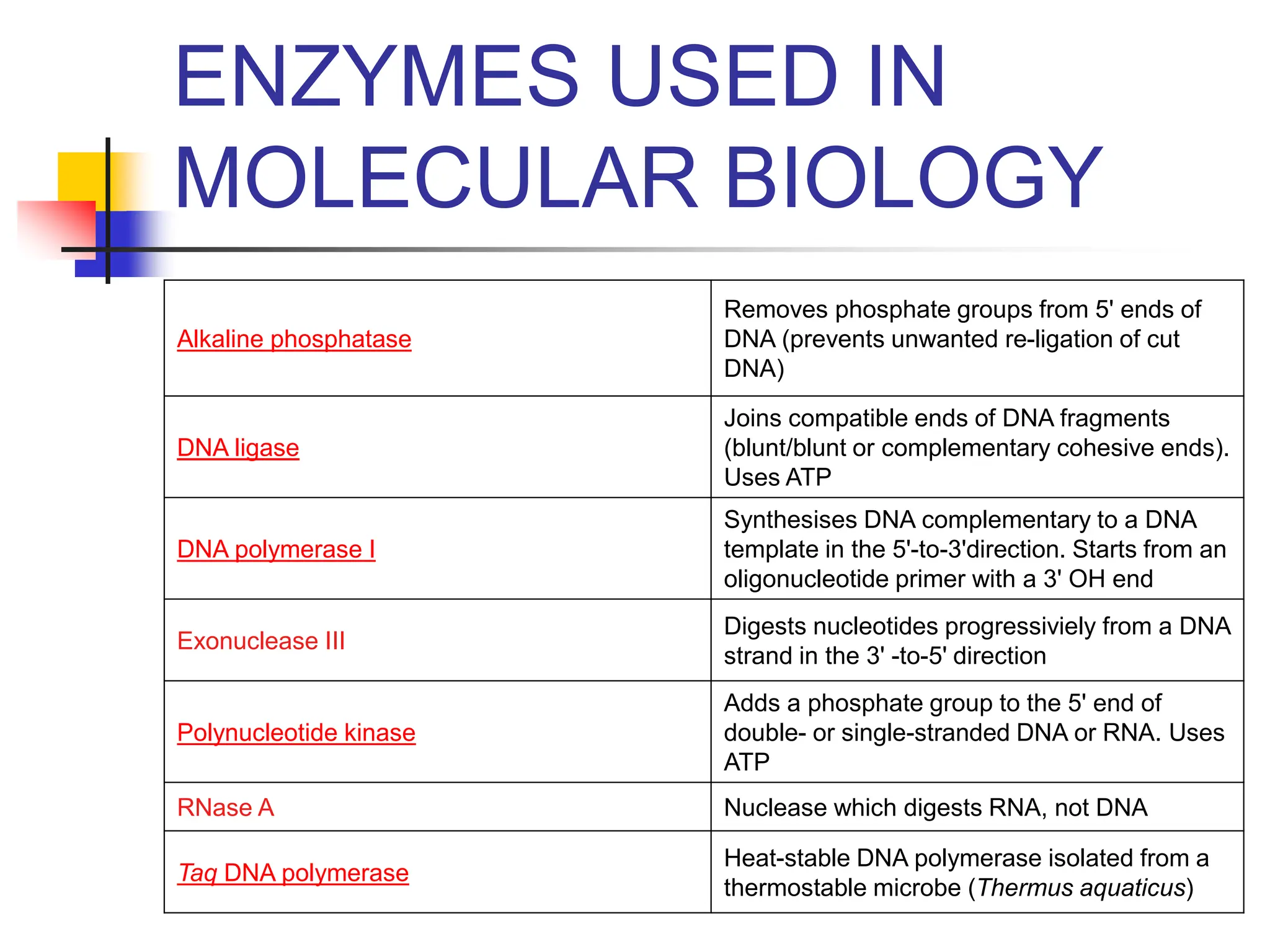
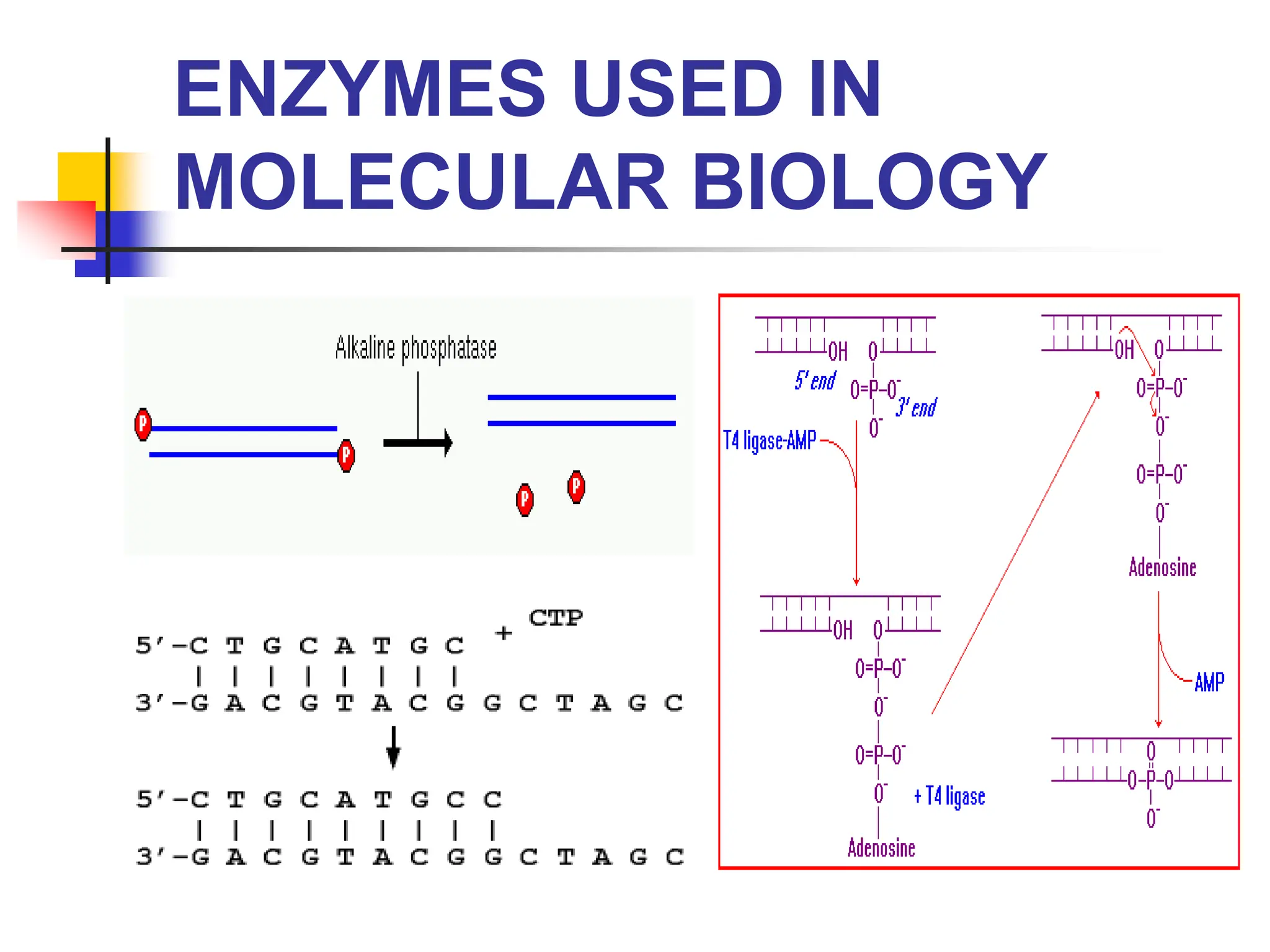
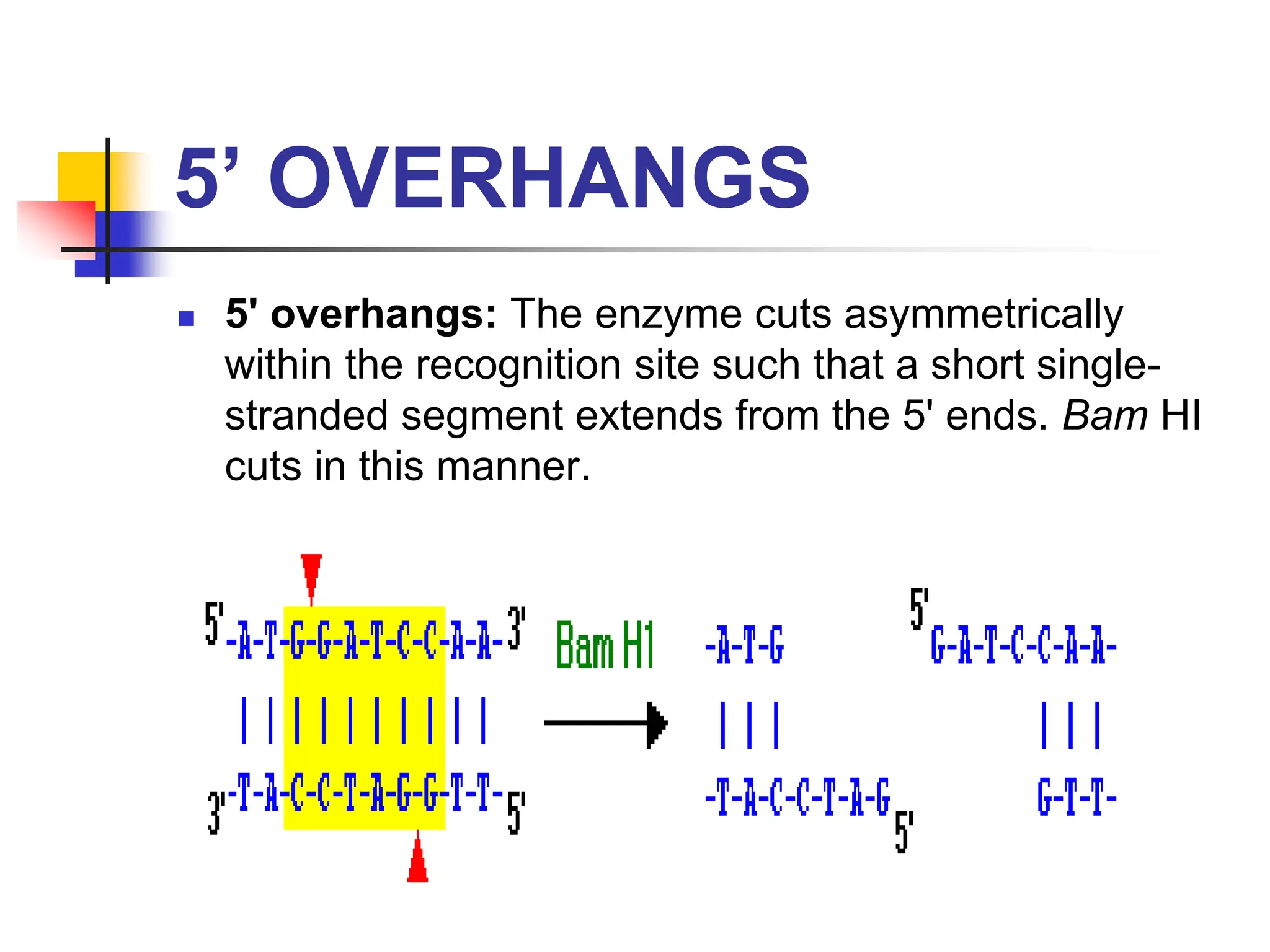
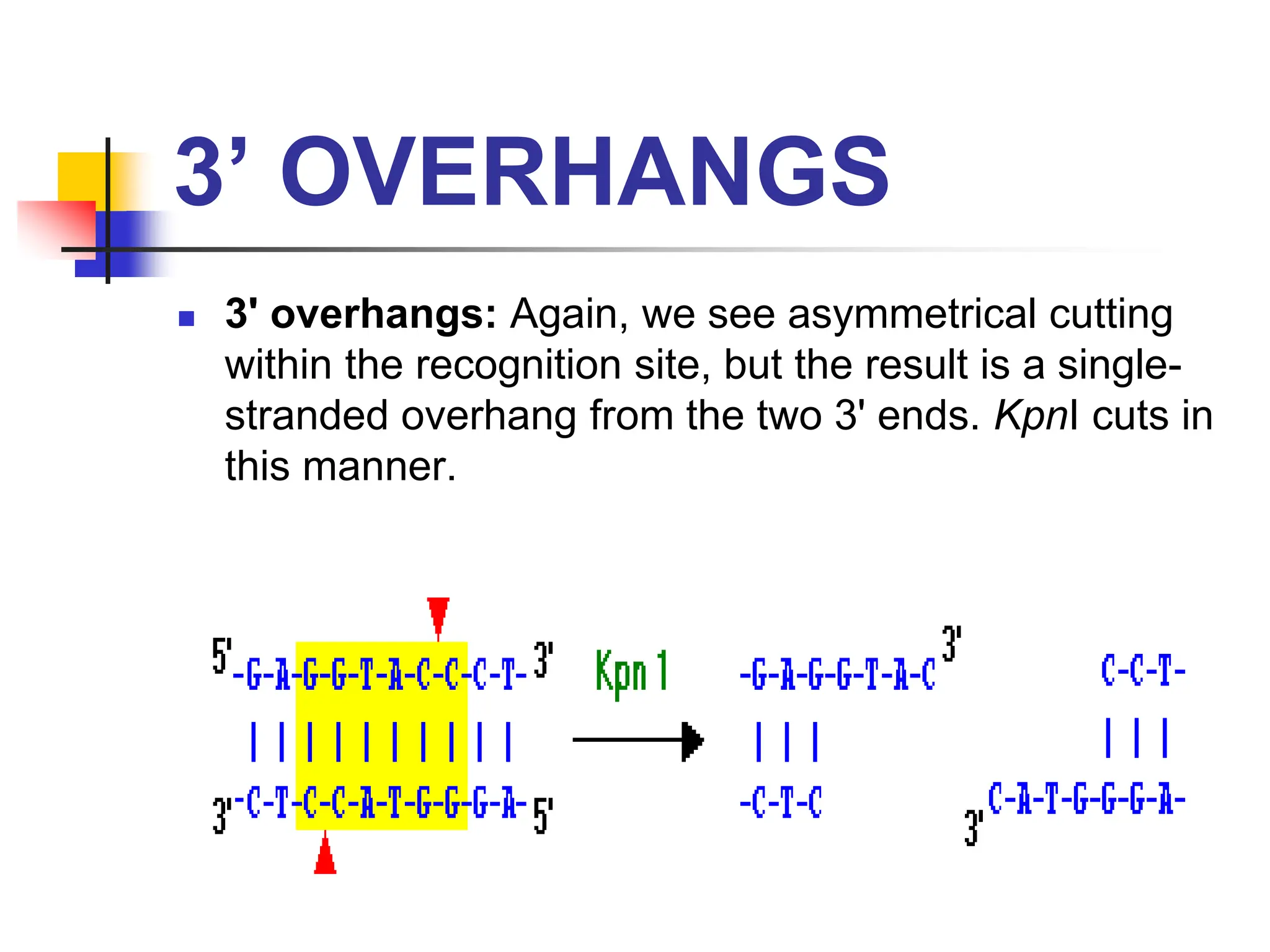
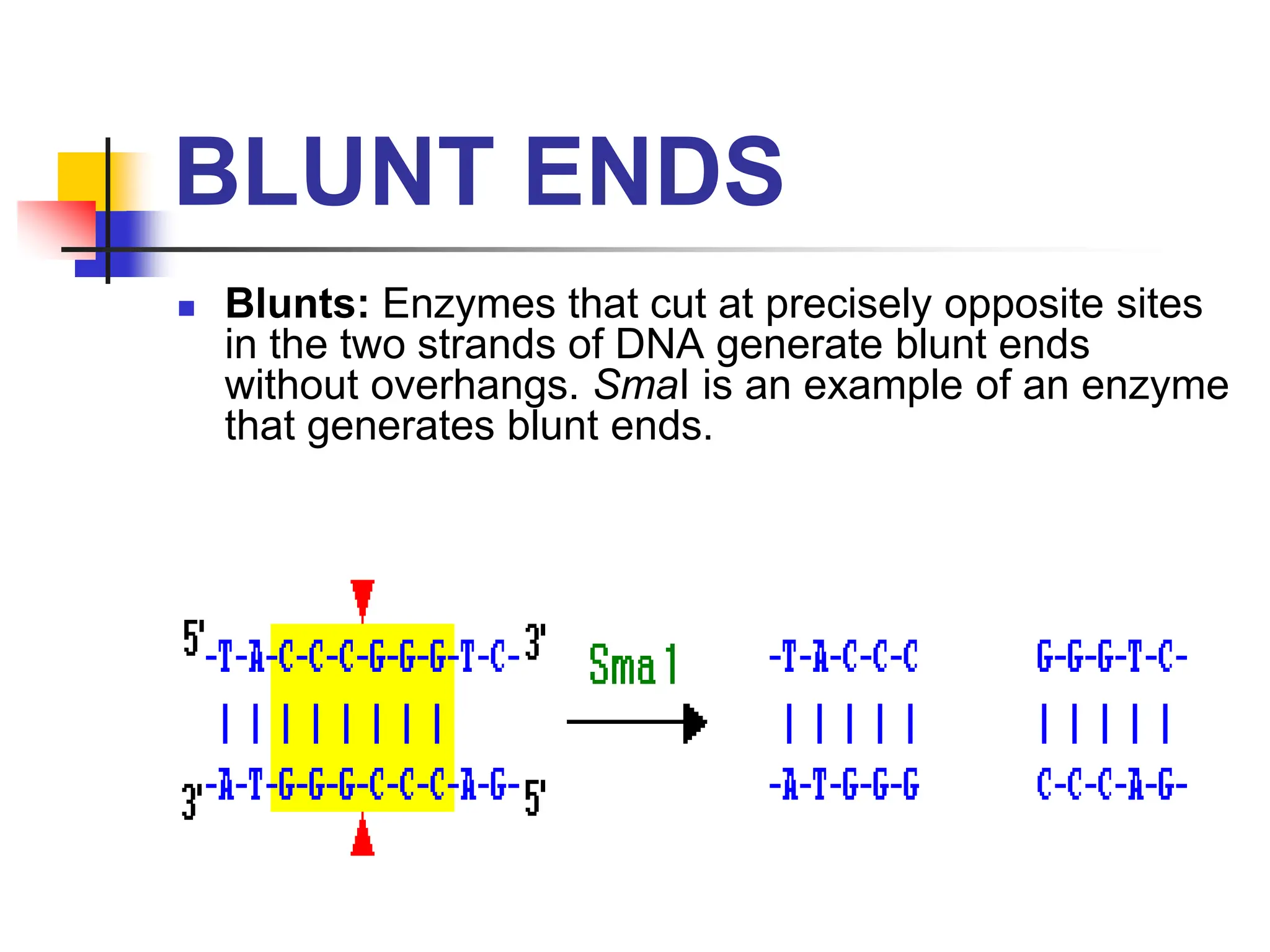
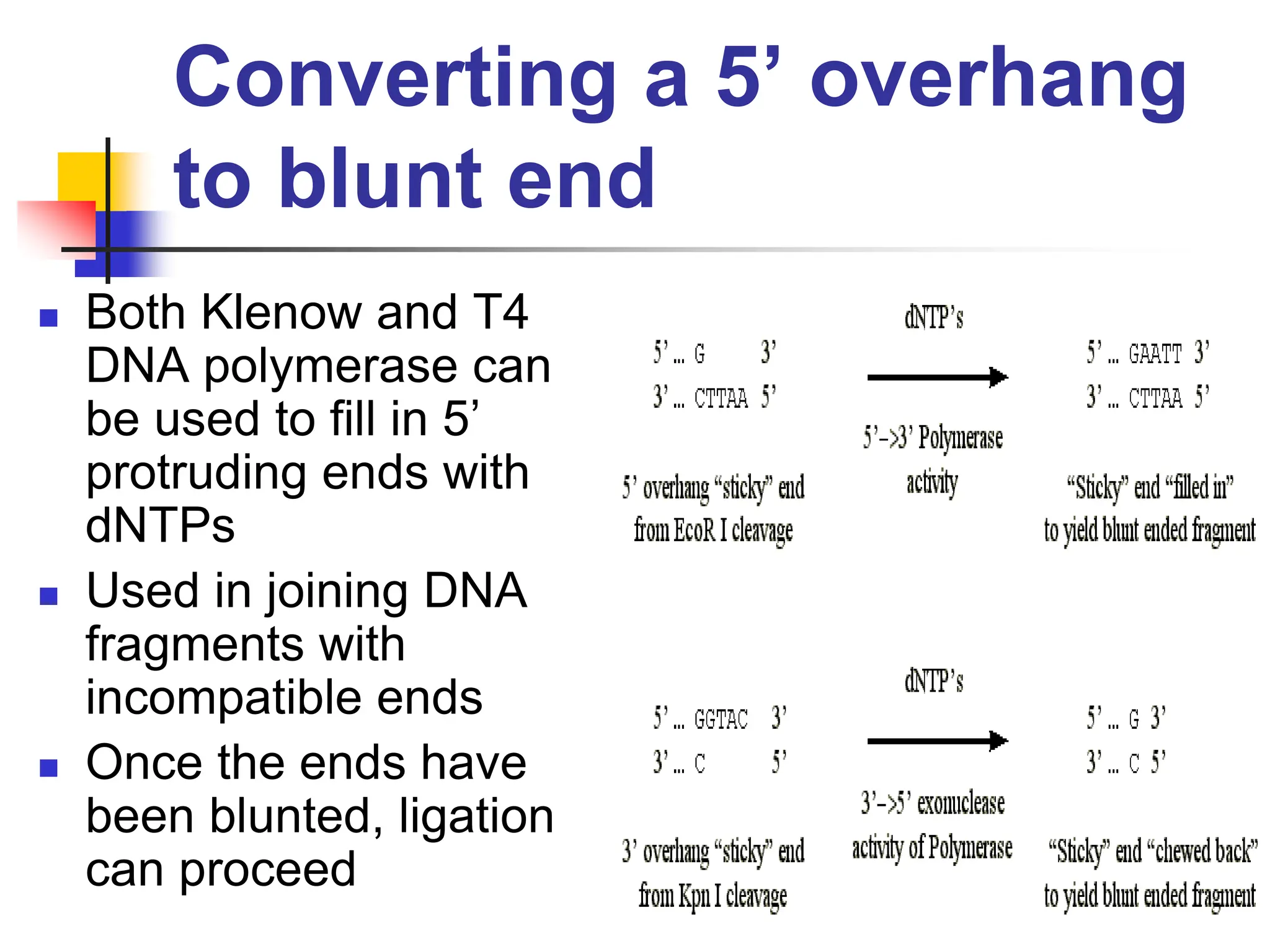
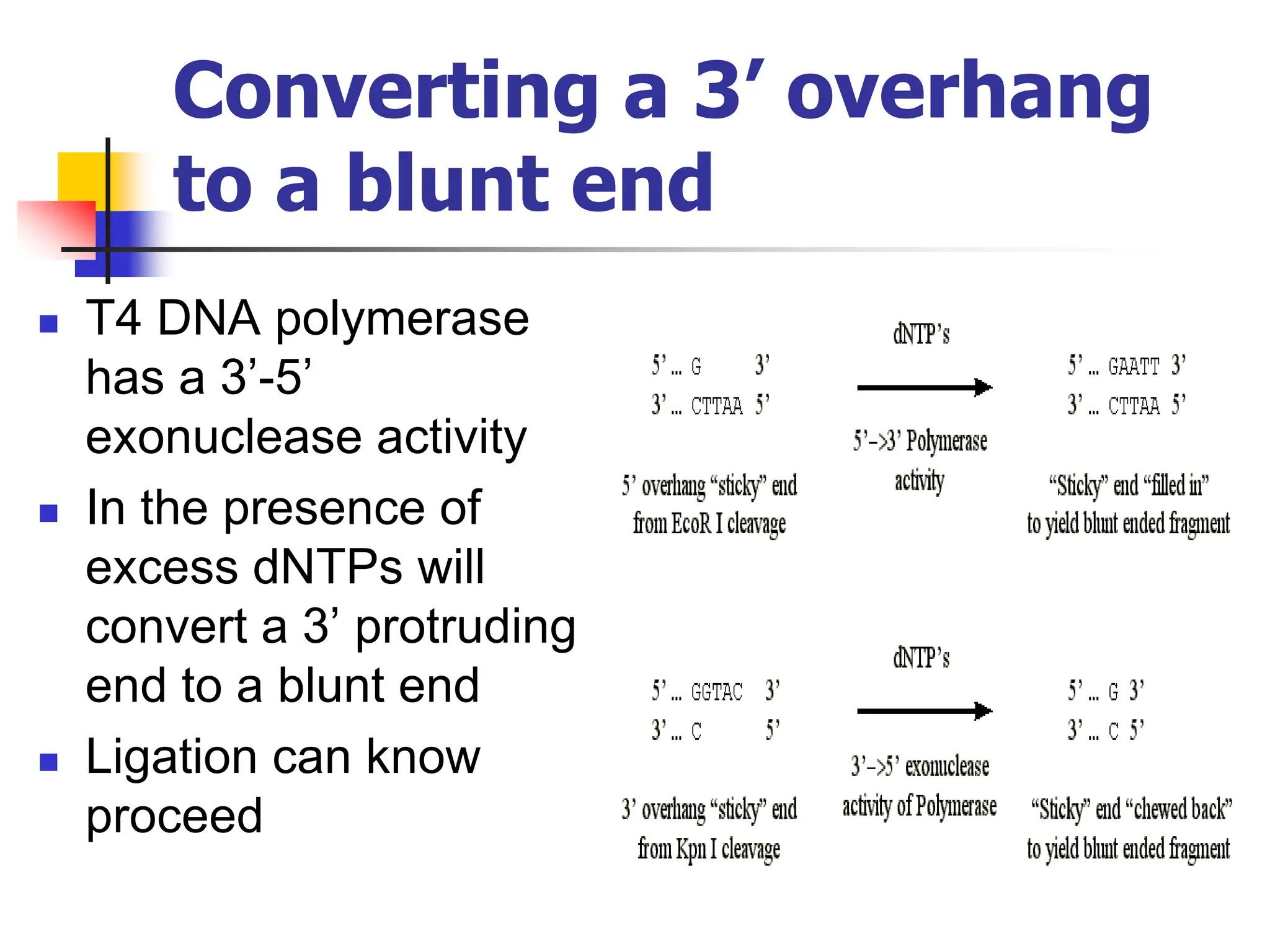
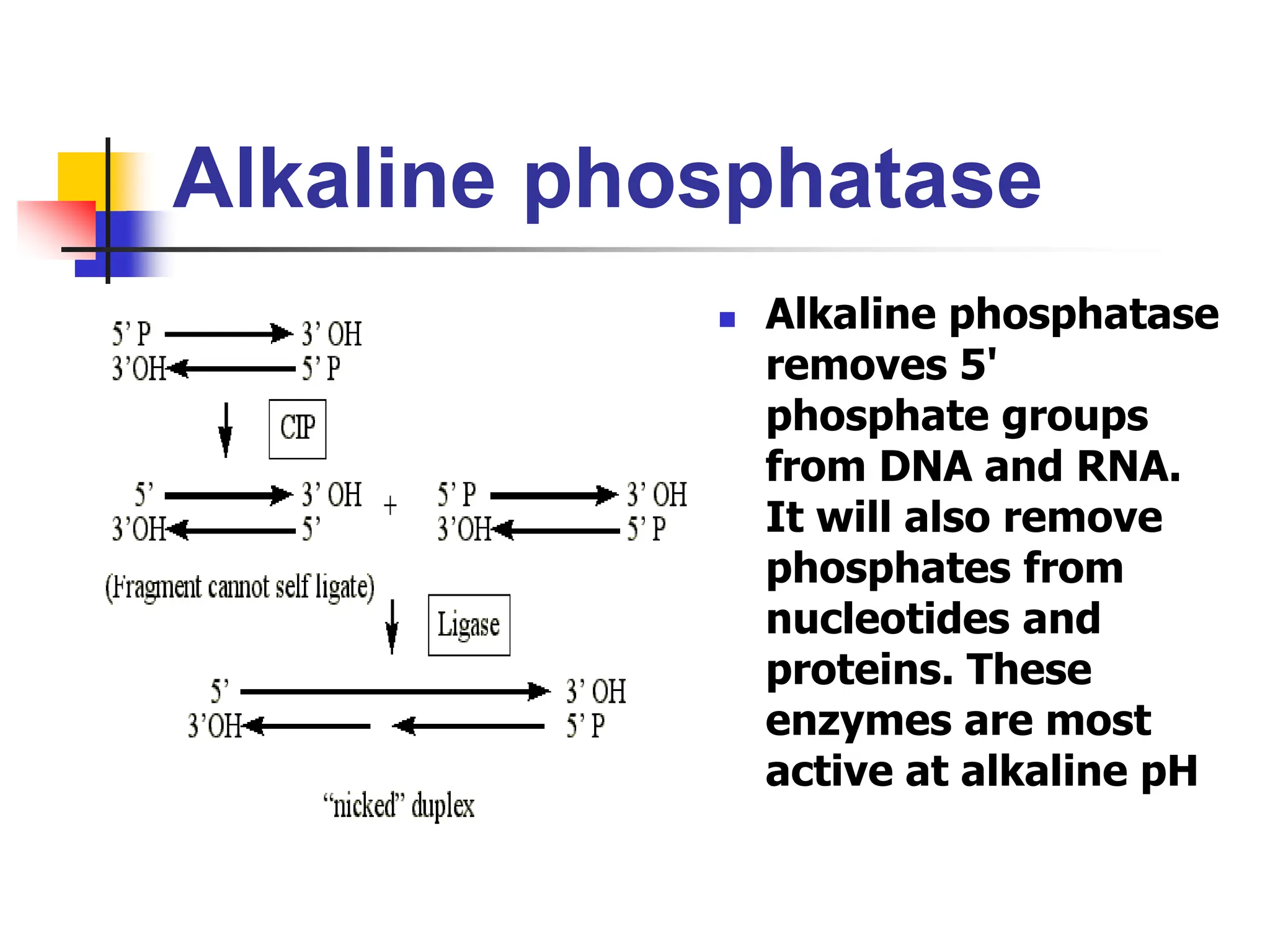
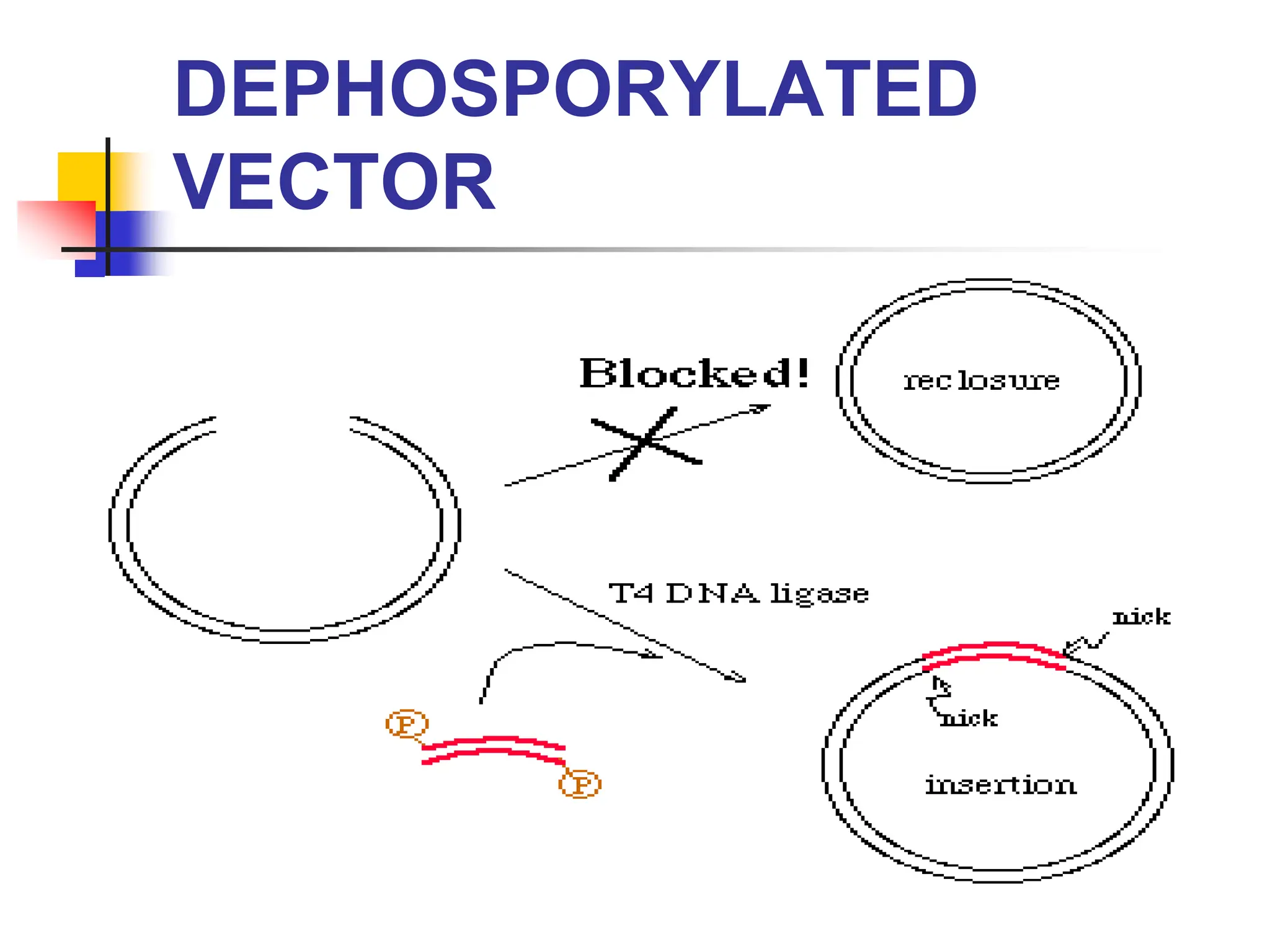
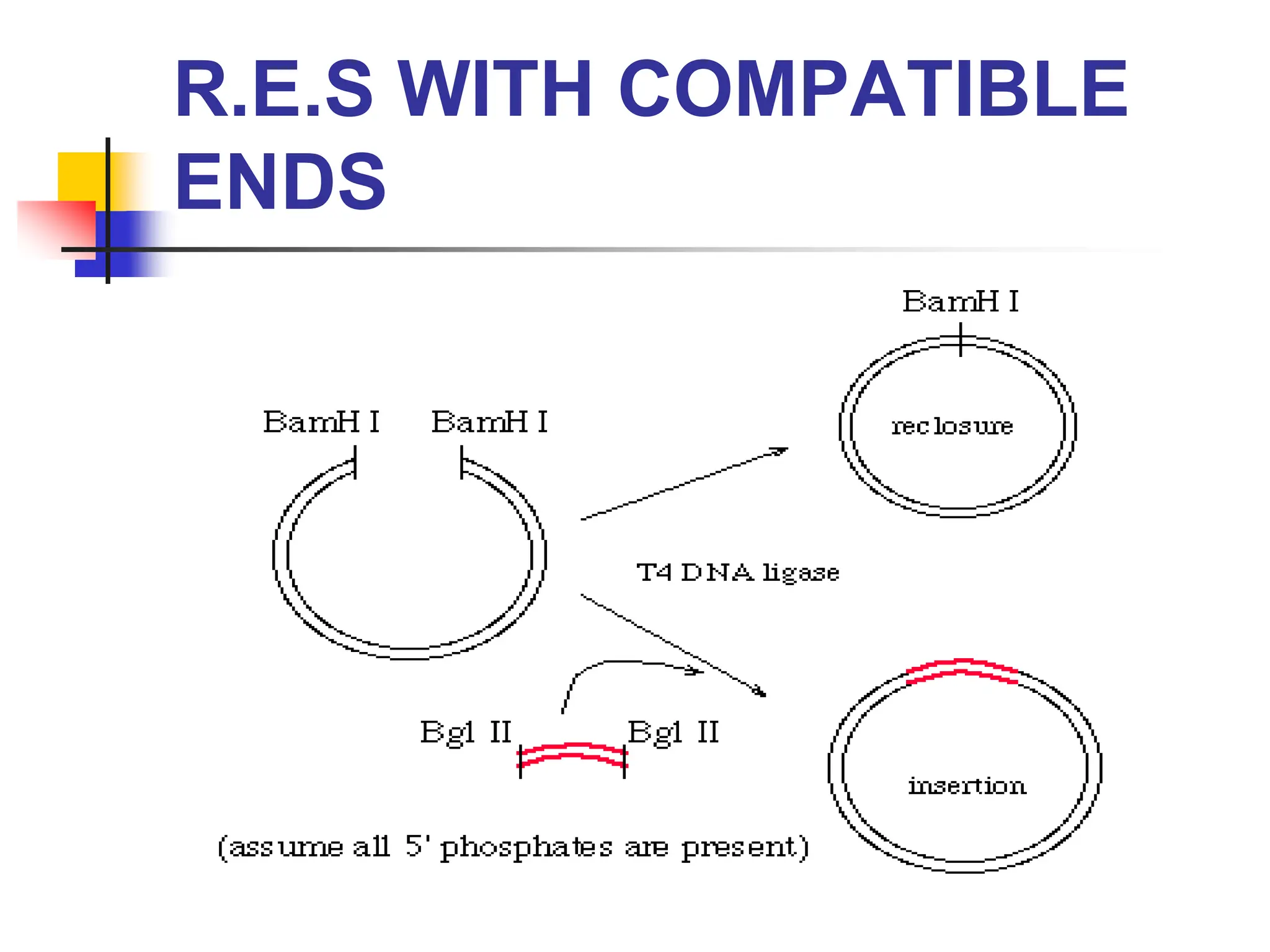
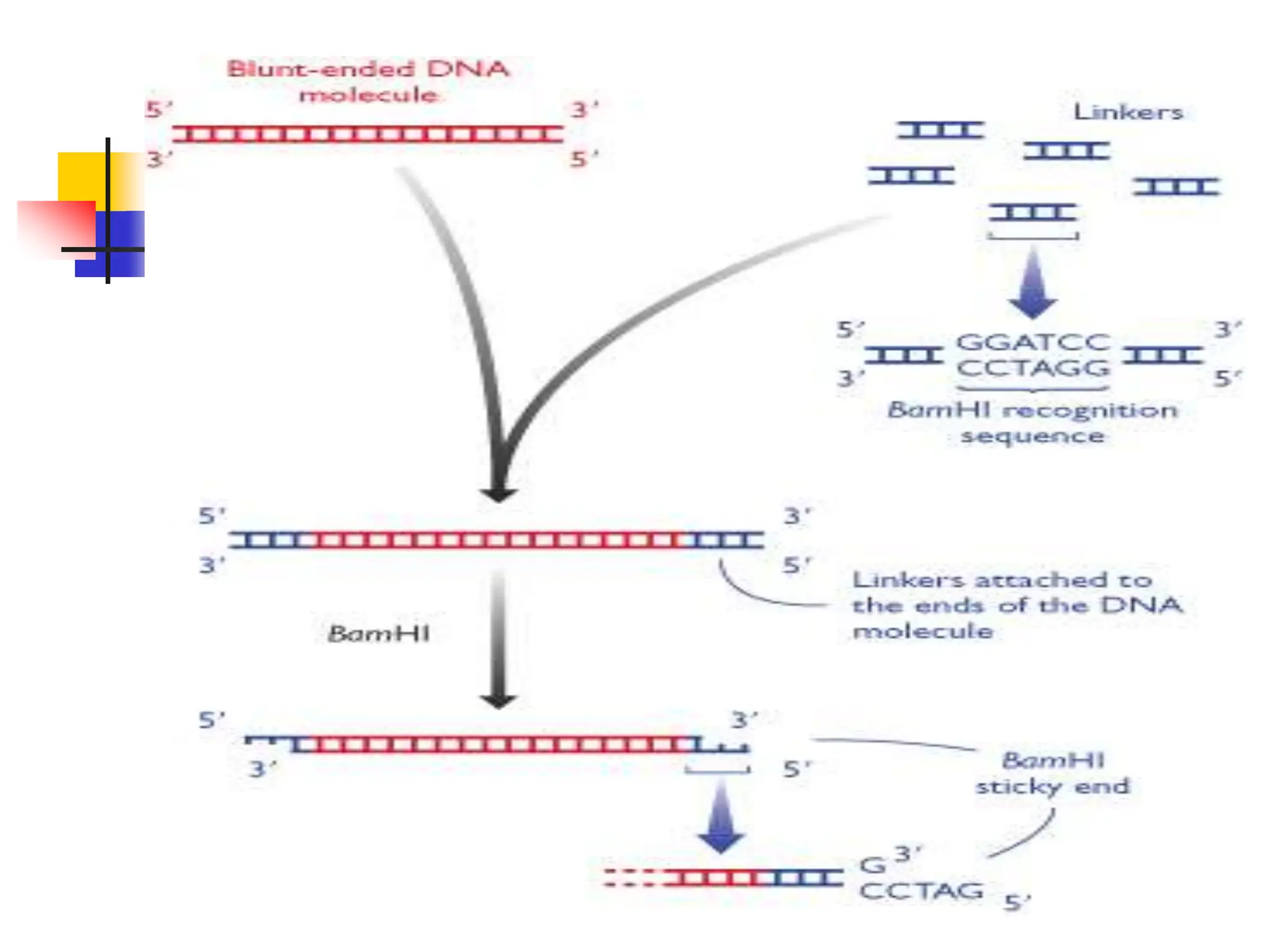
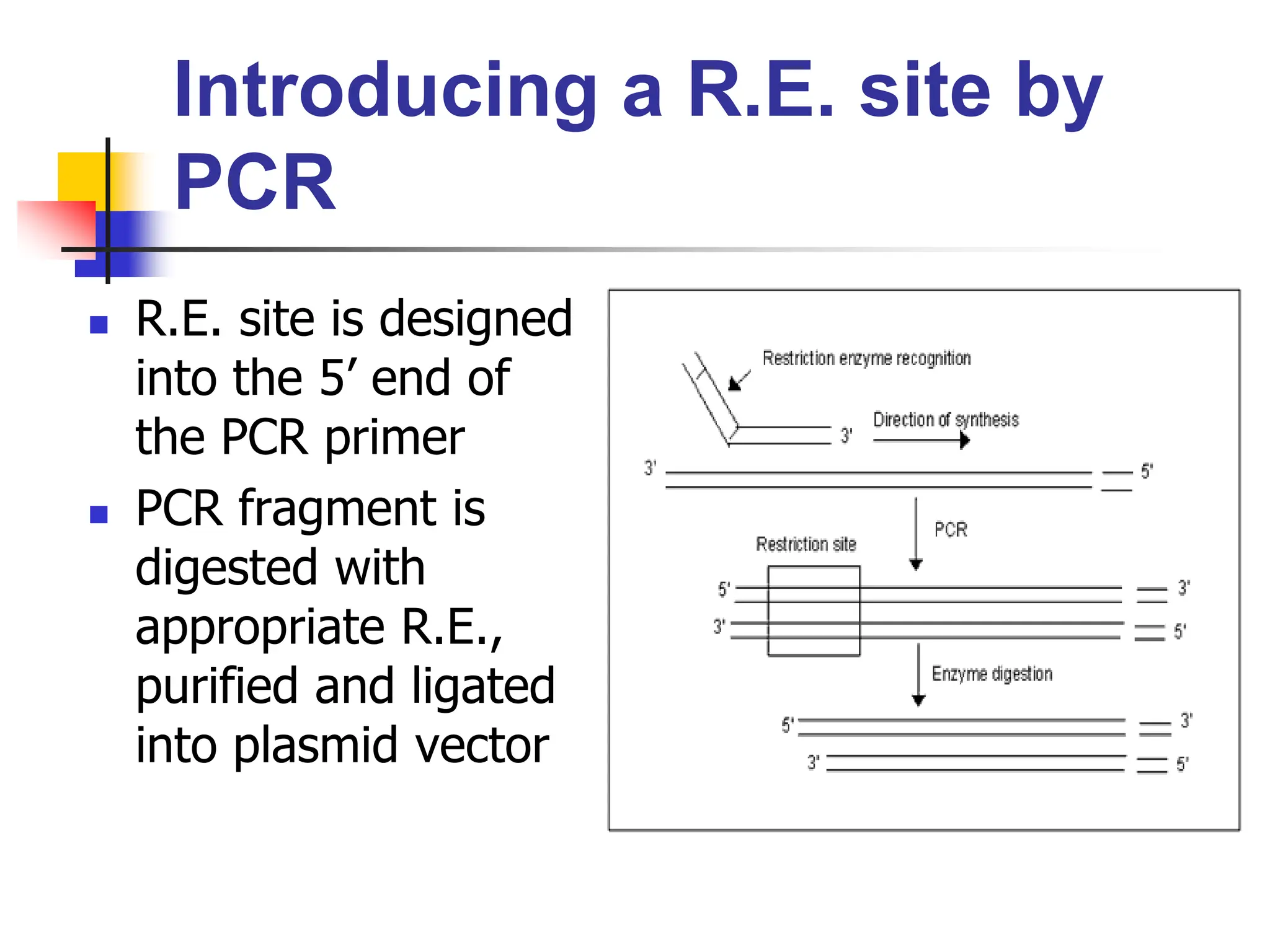
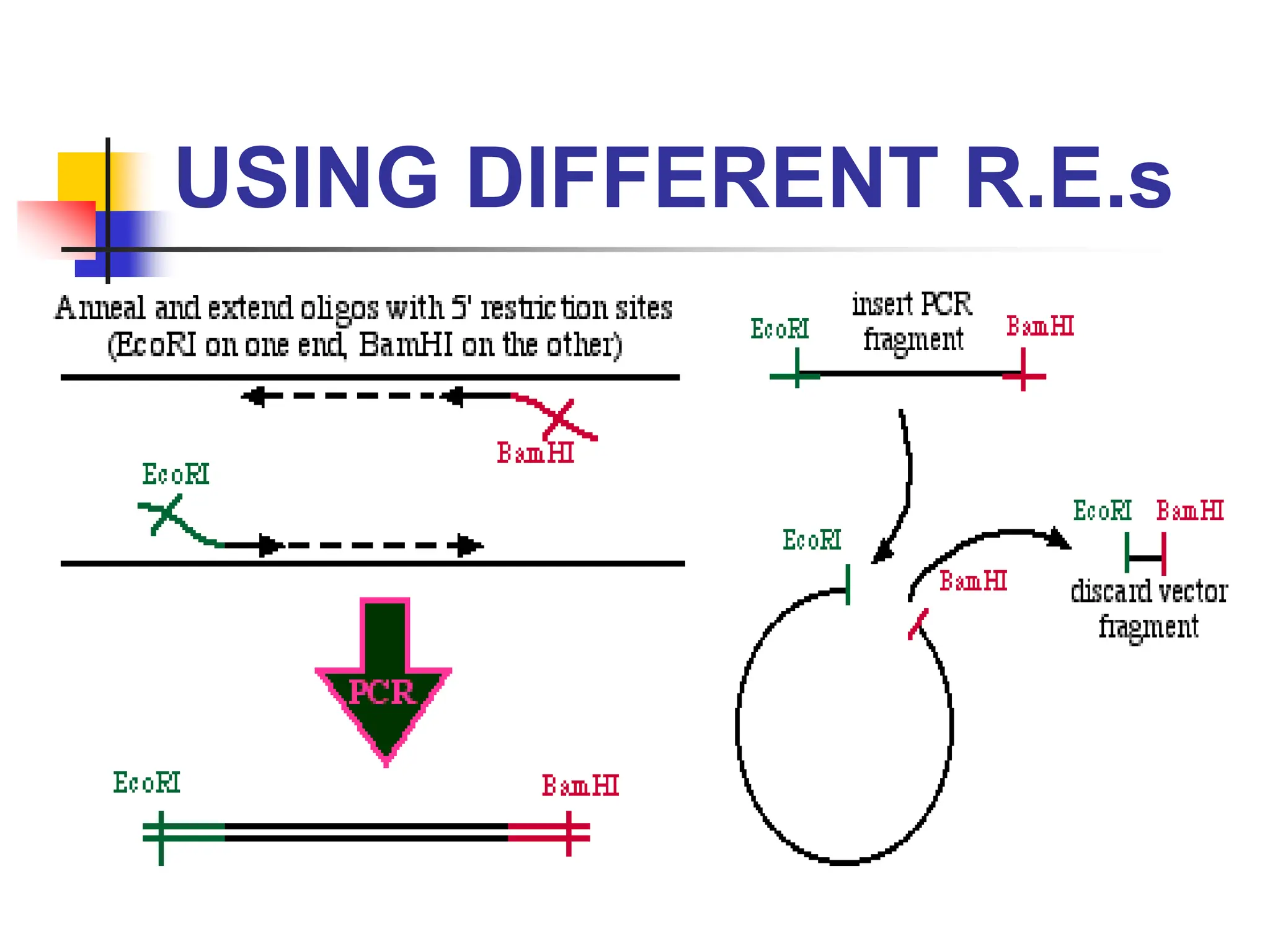
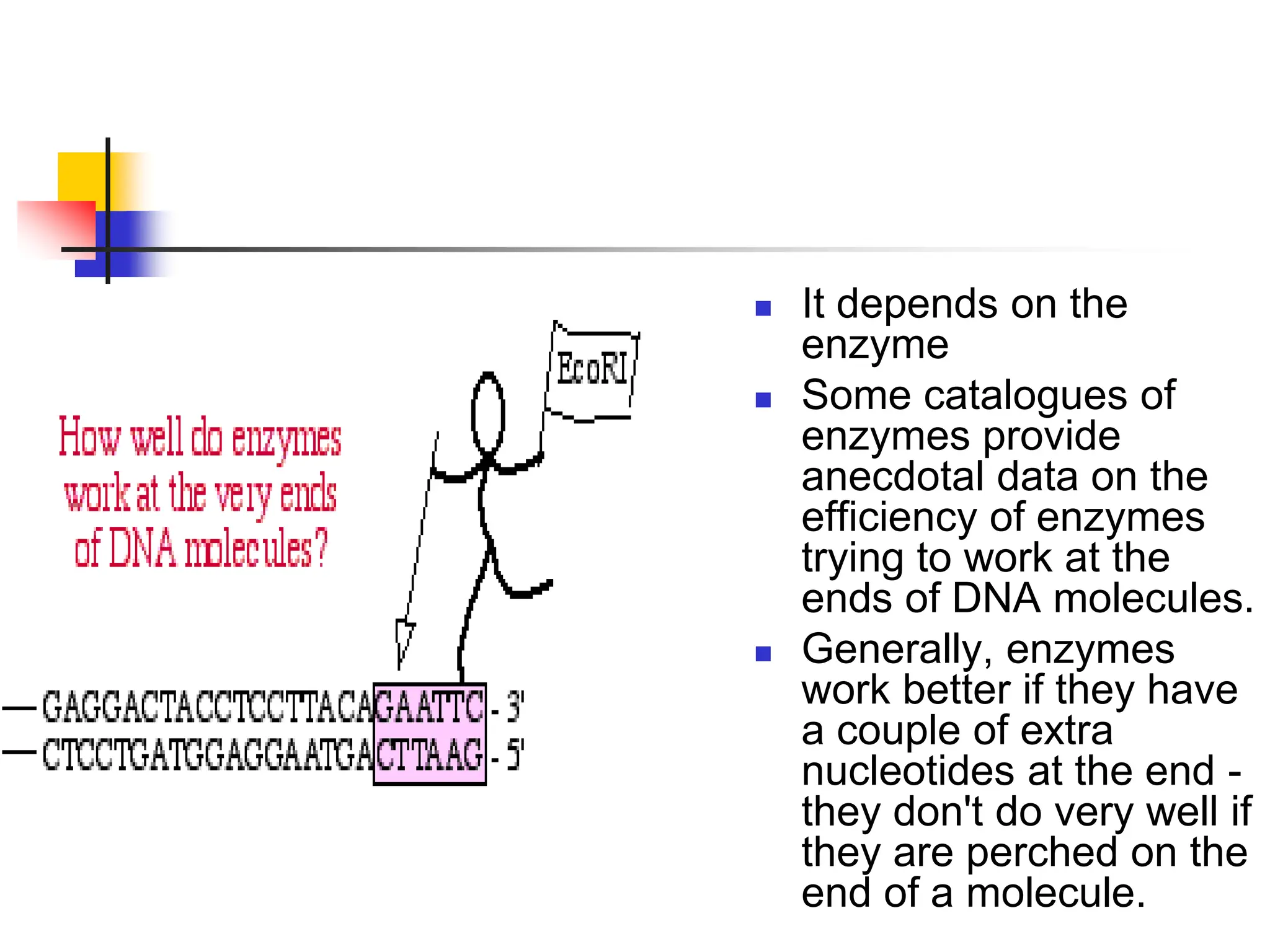
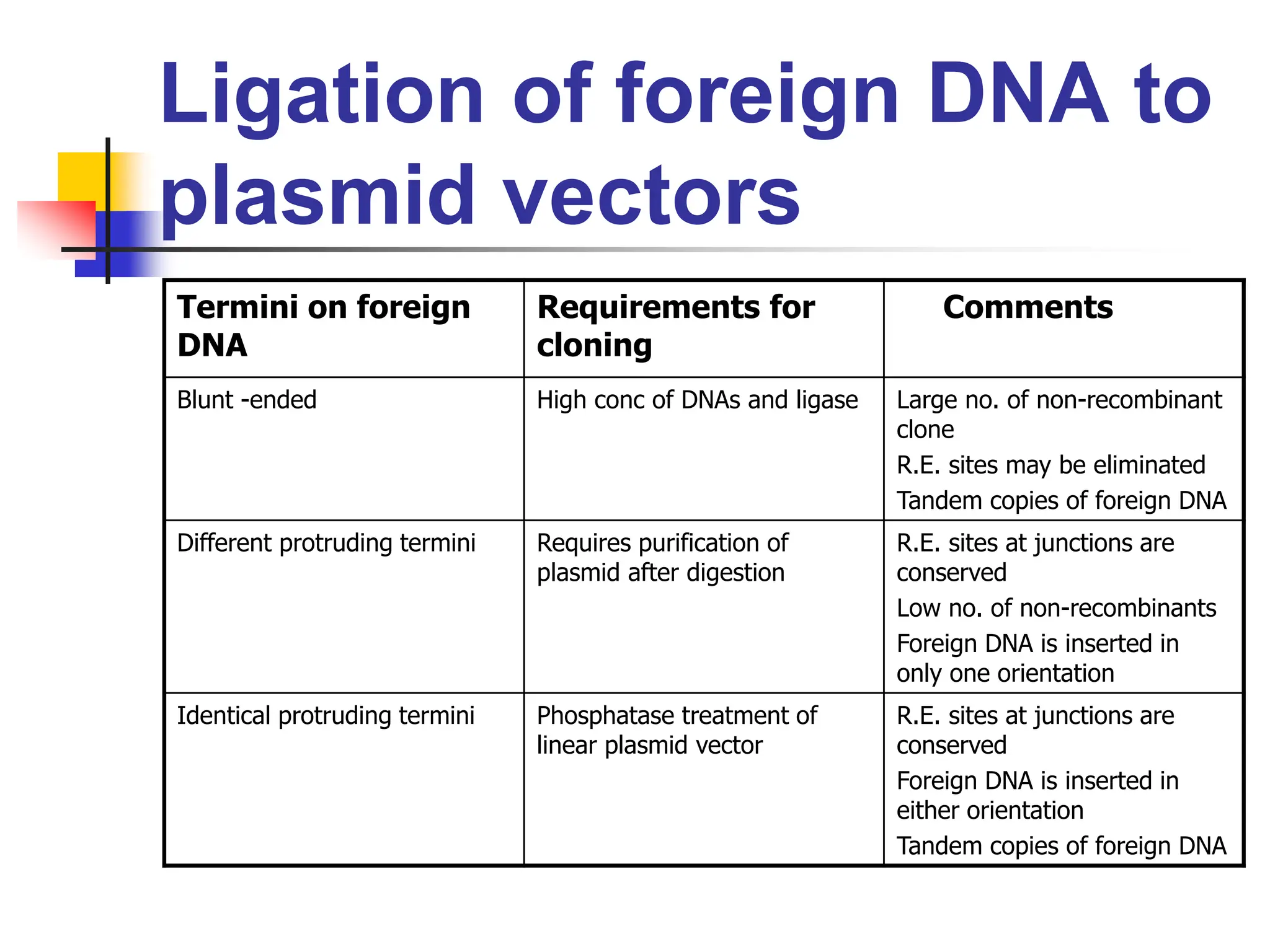
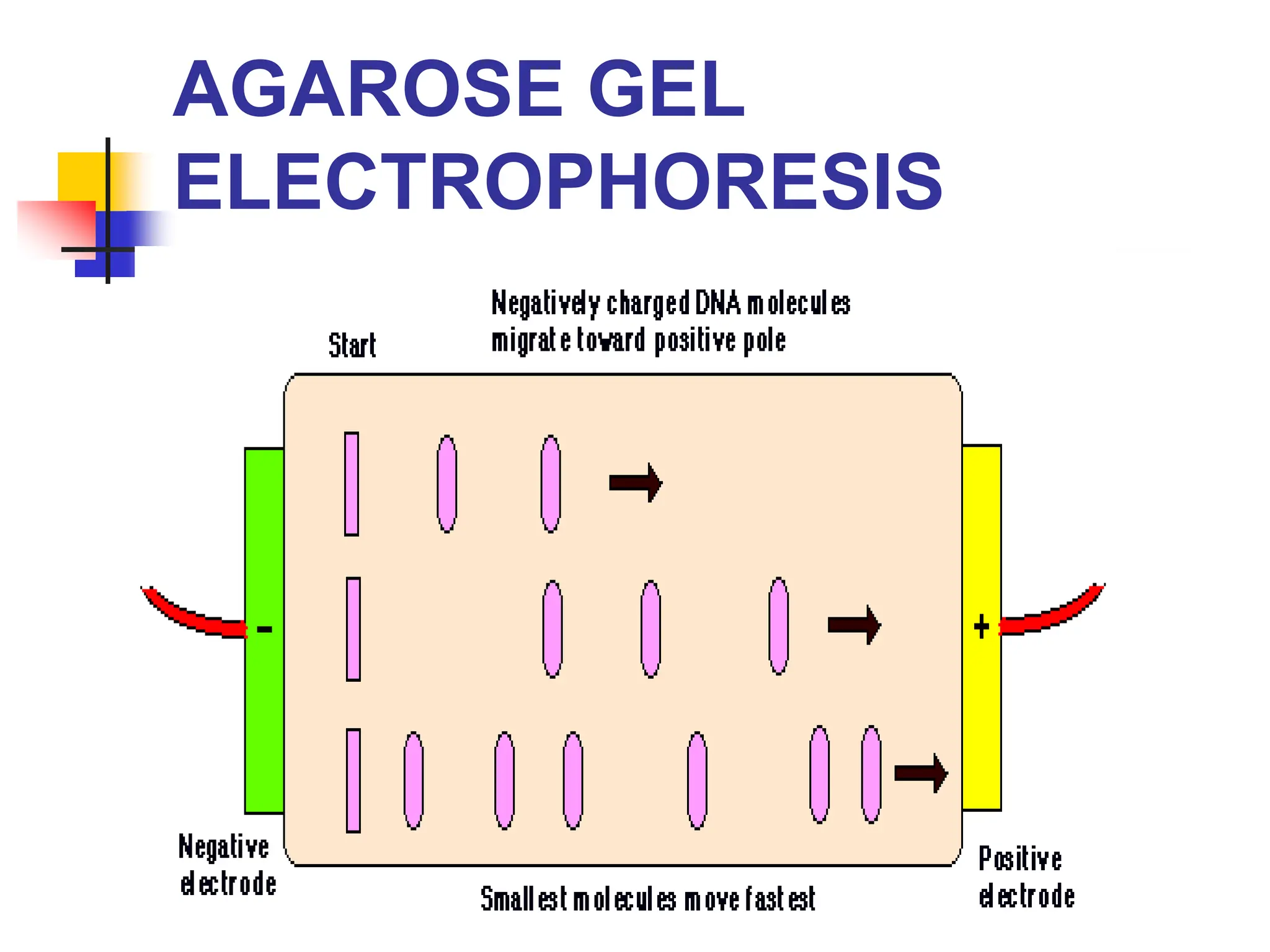
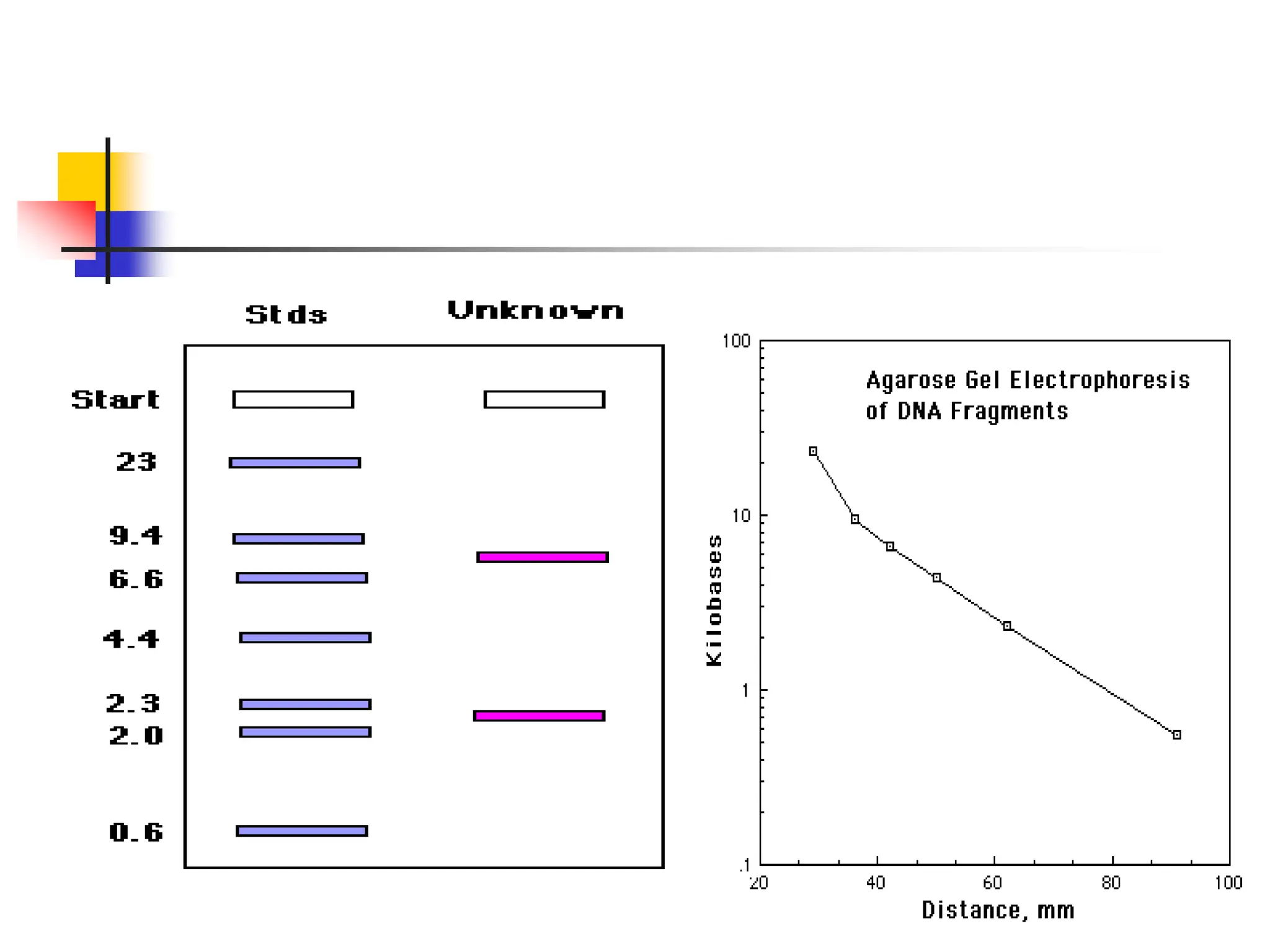
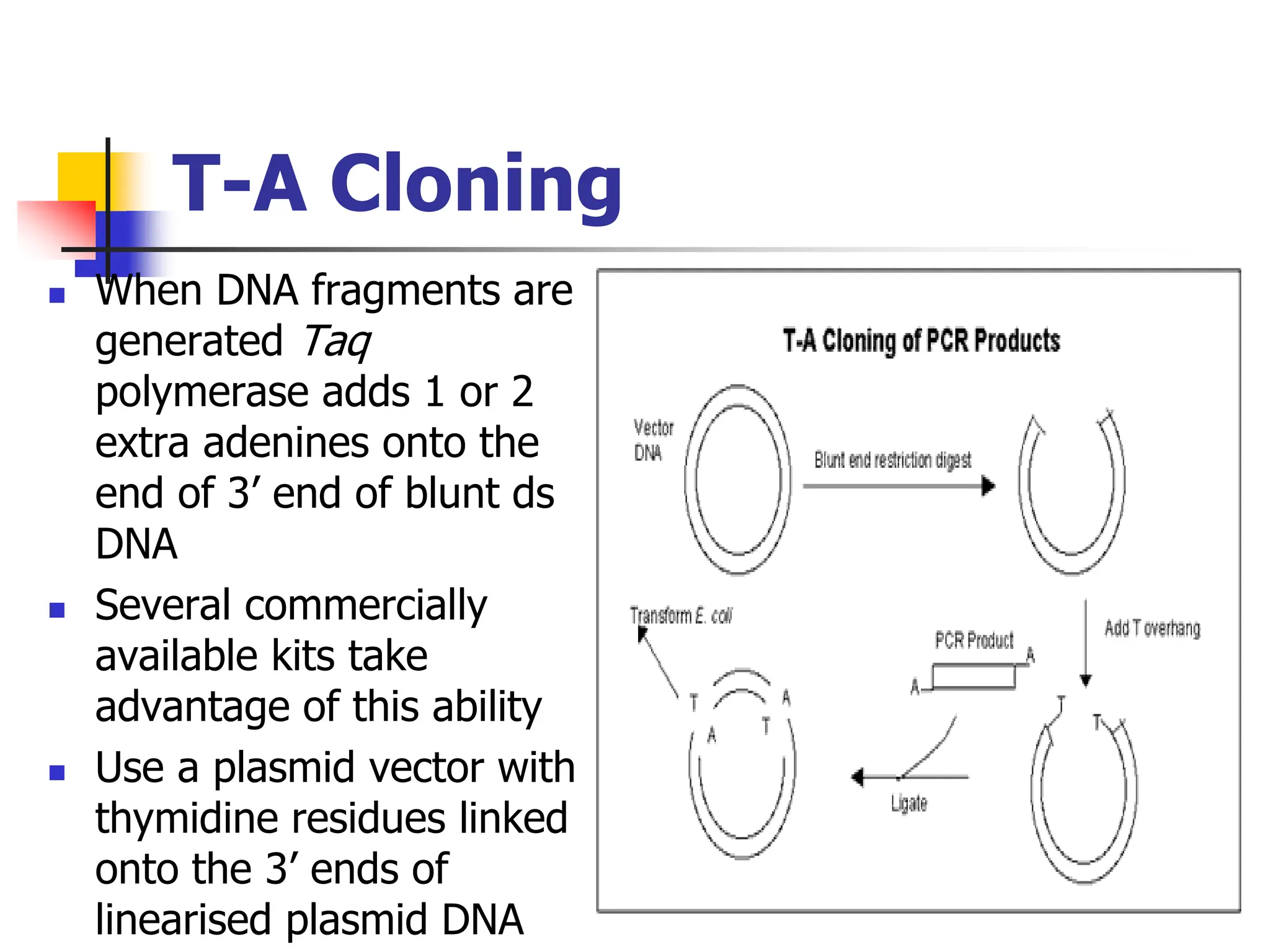
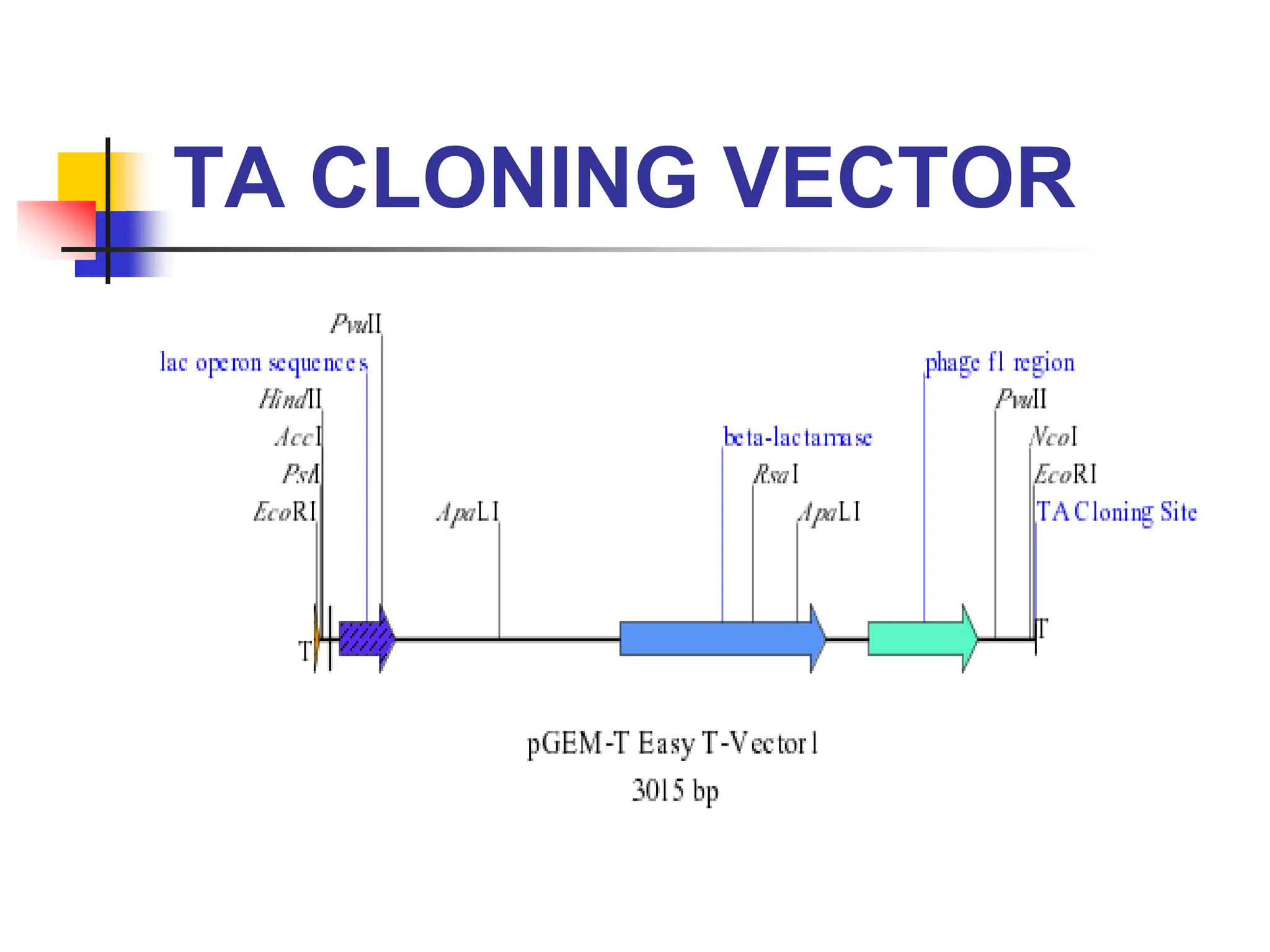
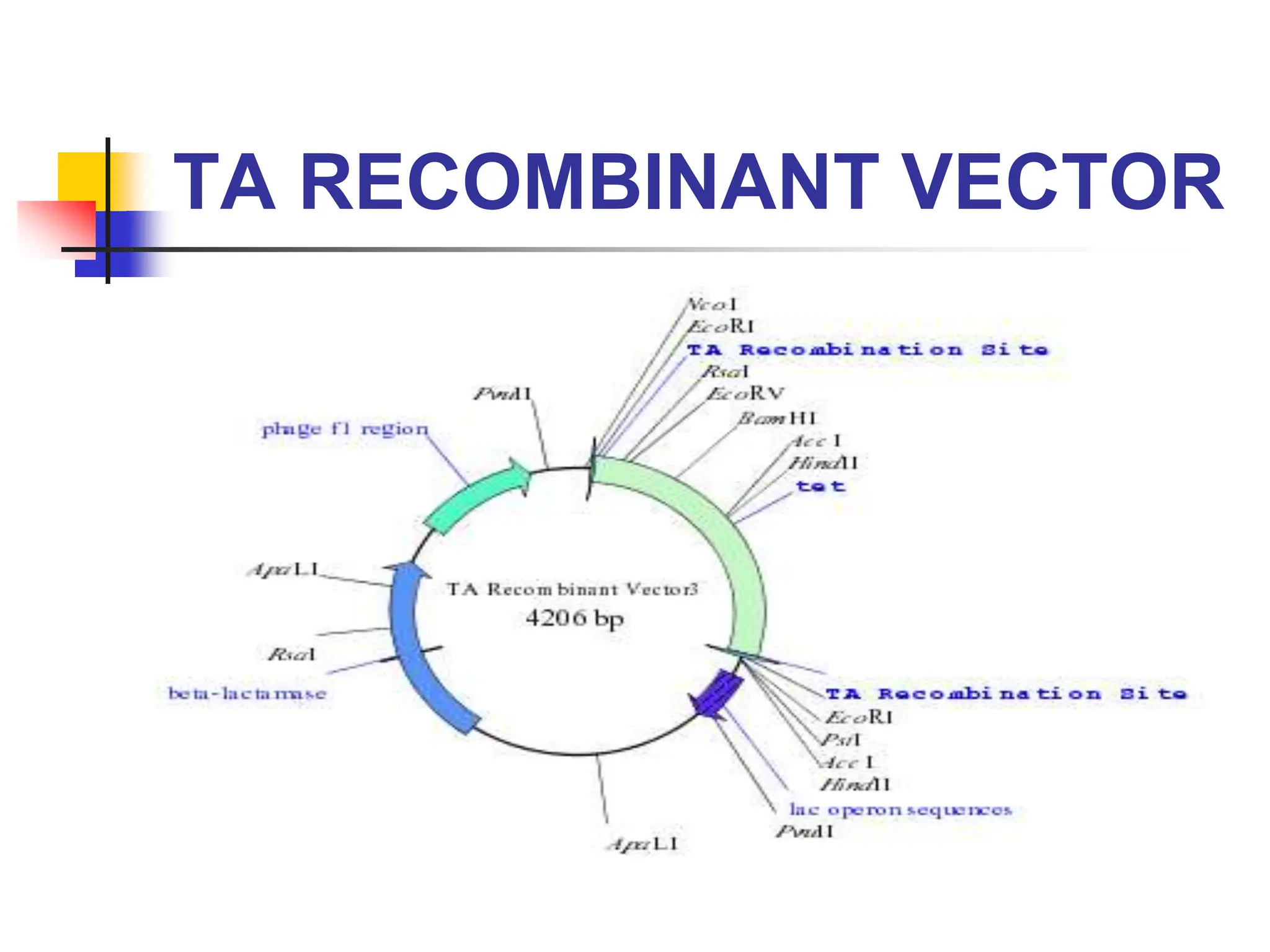
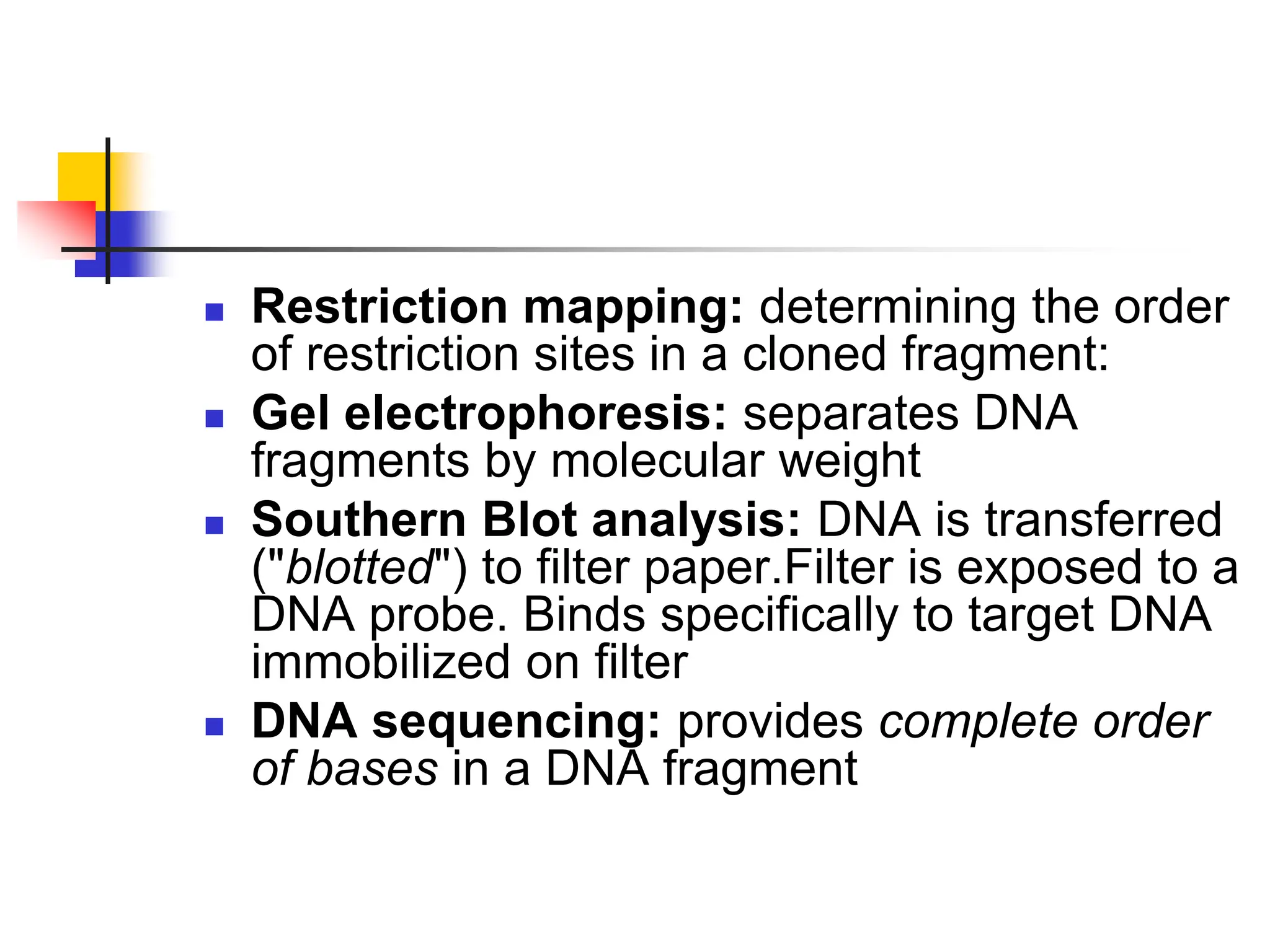
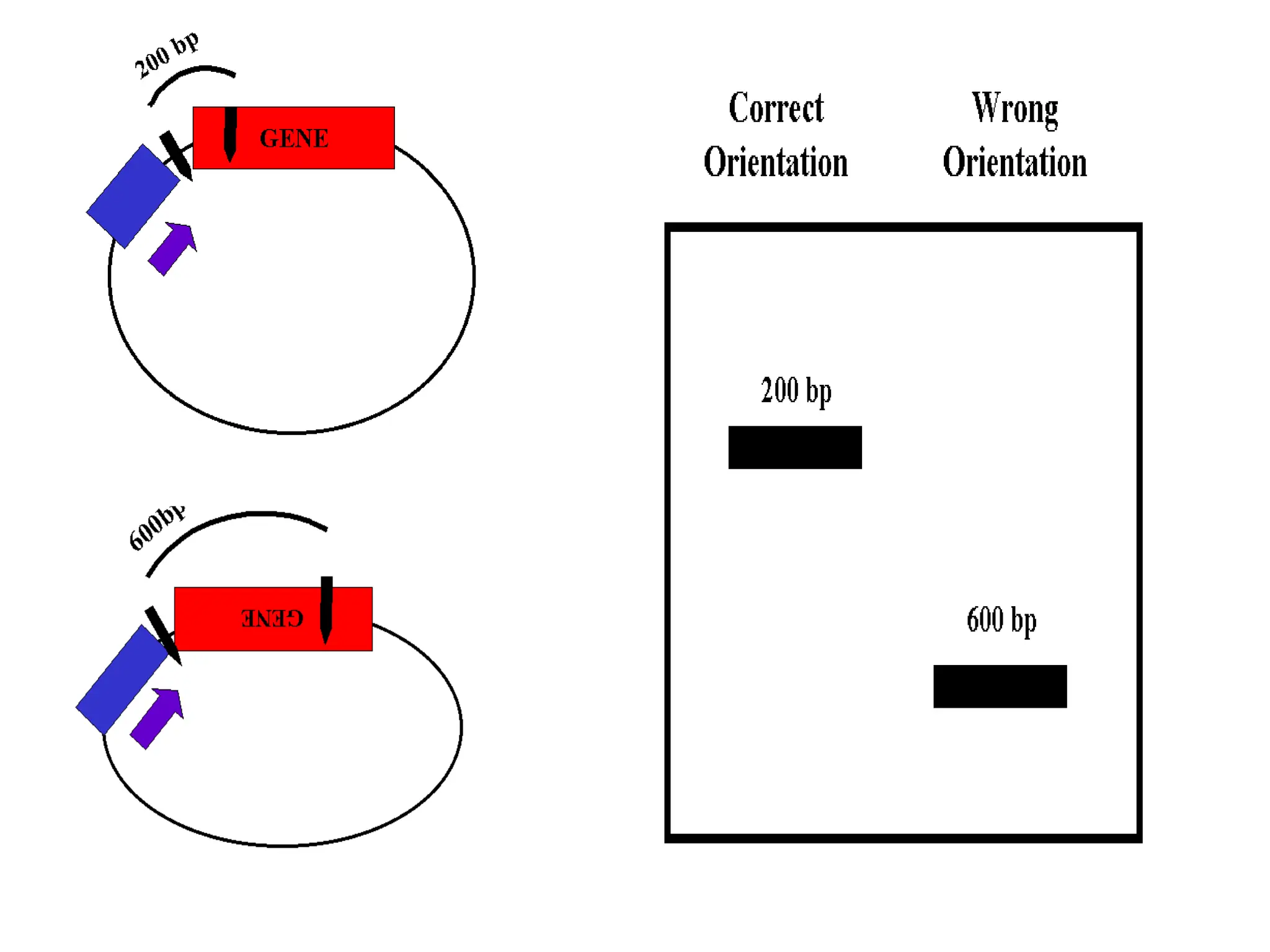
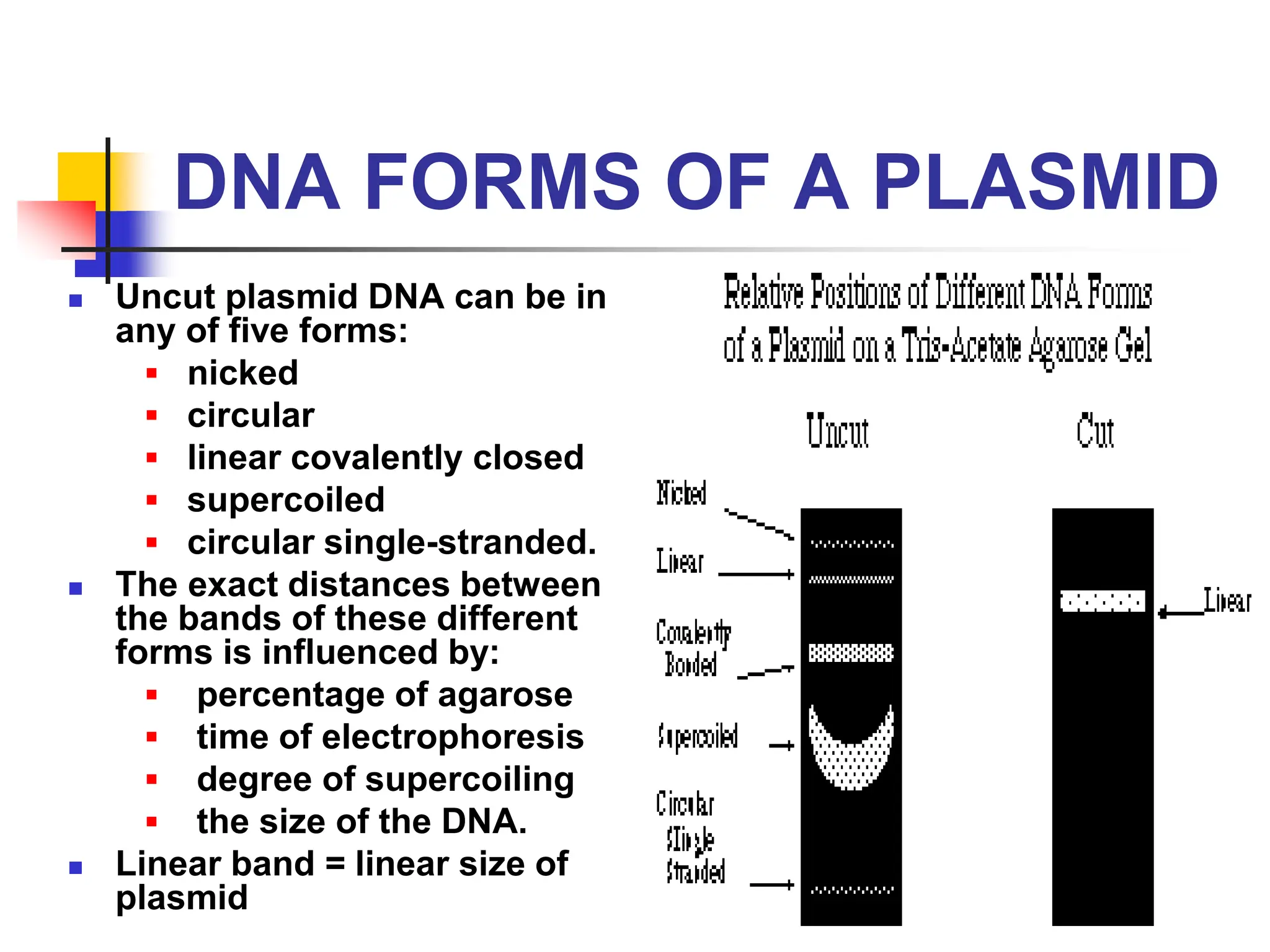
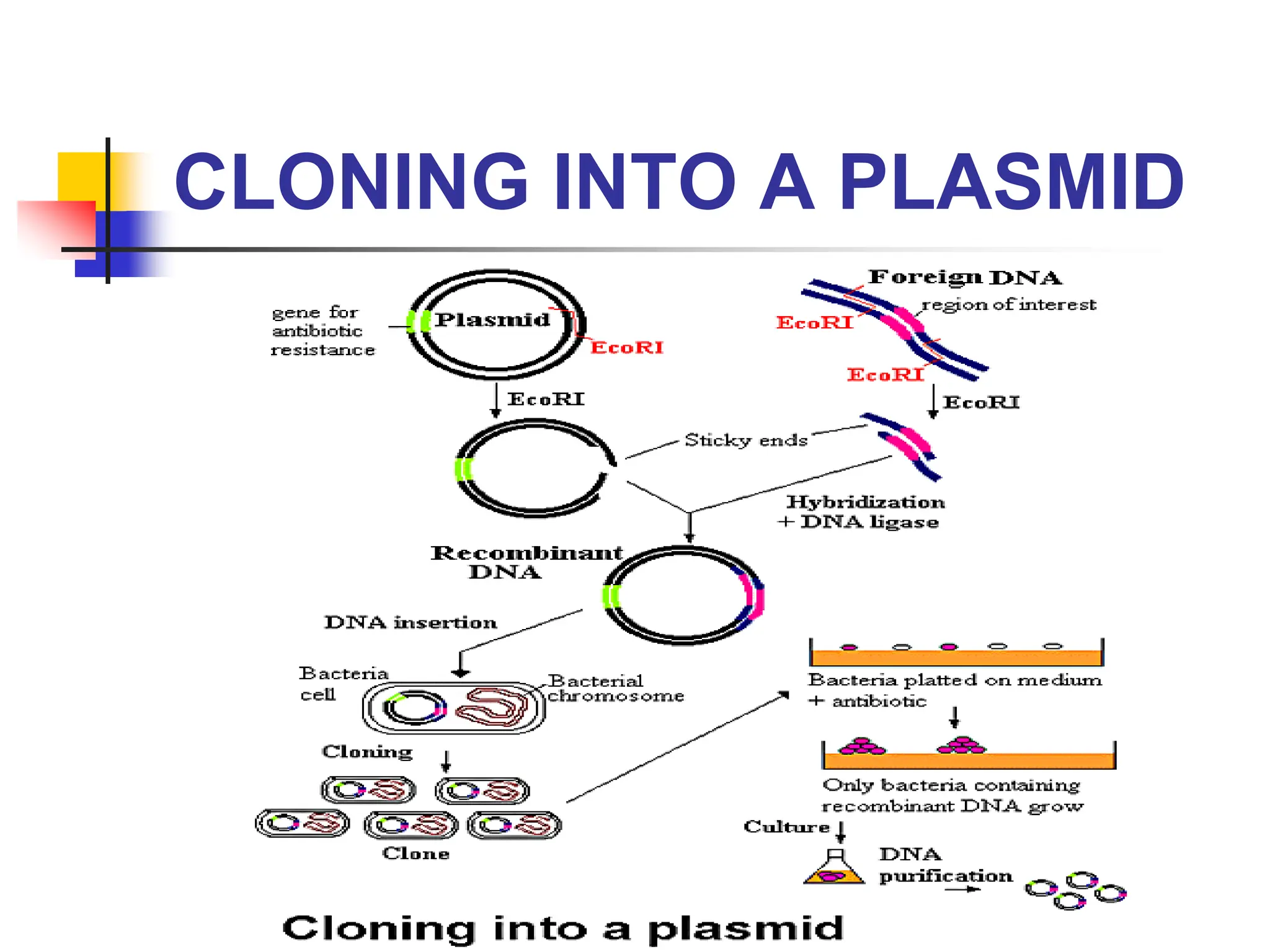
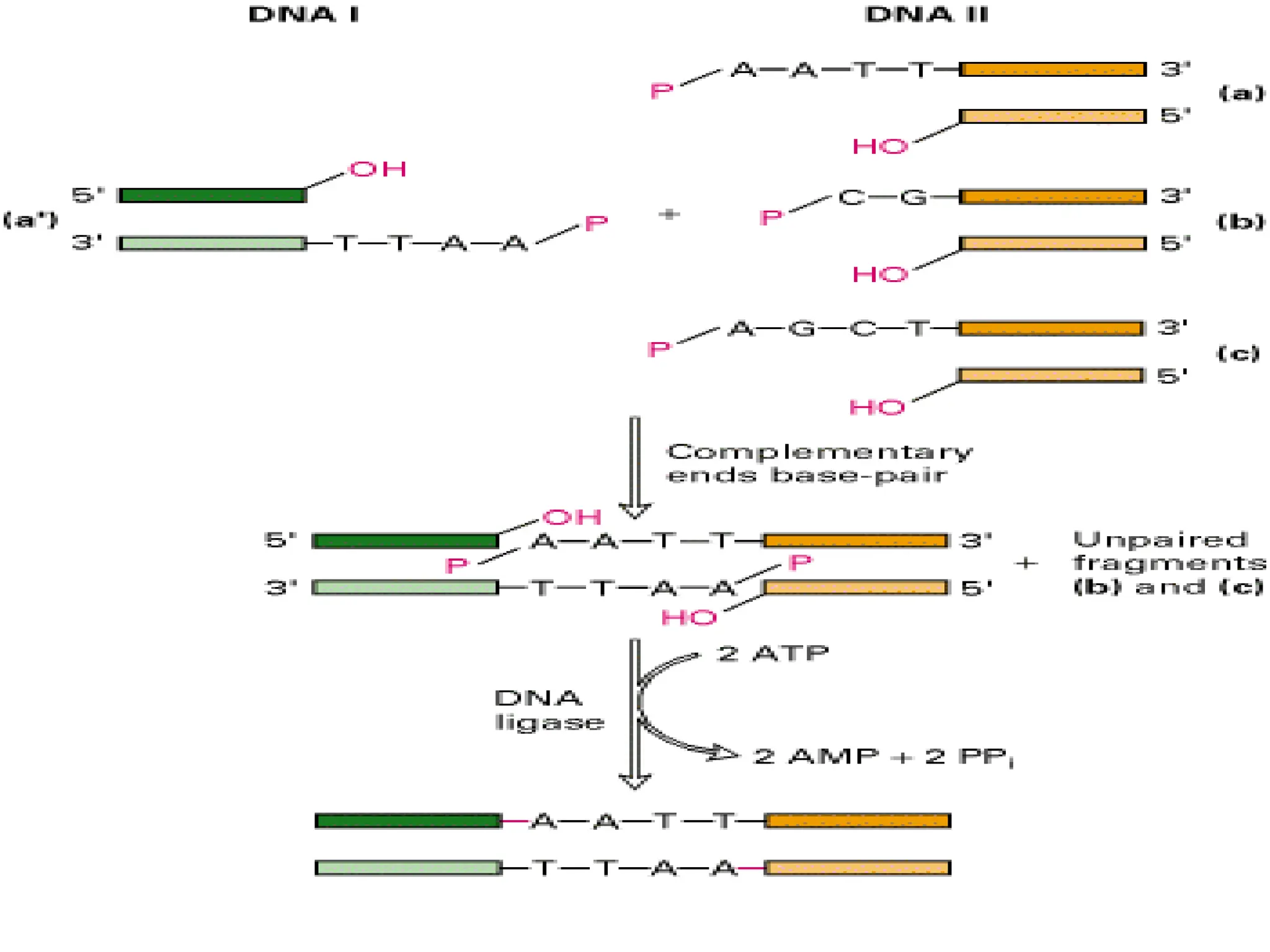
This document discusses principles of DNA cloning including DNA cloning techniques, vectors, and cloning strategies. DNA cloning allows for amplification of specific DNA fragments and involves inserting DNA fragments into vectors, such as plasmids, for propagation in host cells. Key steps in plasmid cloning include restriction enzyme digestion of the DNA fragment and vector, ligation of the fragment into the vector, transformation of host cells, and selection of cells containing the recombinant DNA. Common vectors include plasmids, bacteriophages, cosmids, and yeast artificial chromosomes which can accommodate varying sizes of DNA inserts.