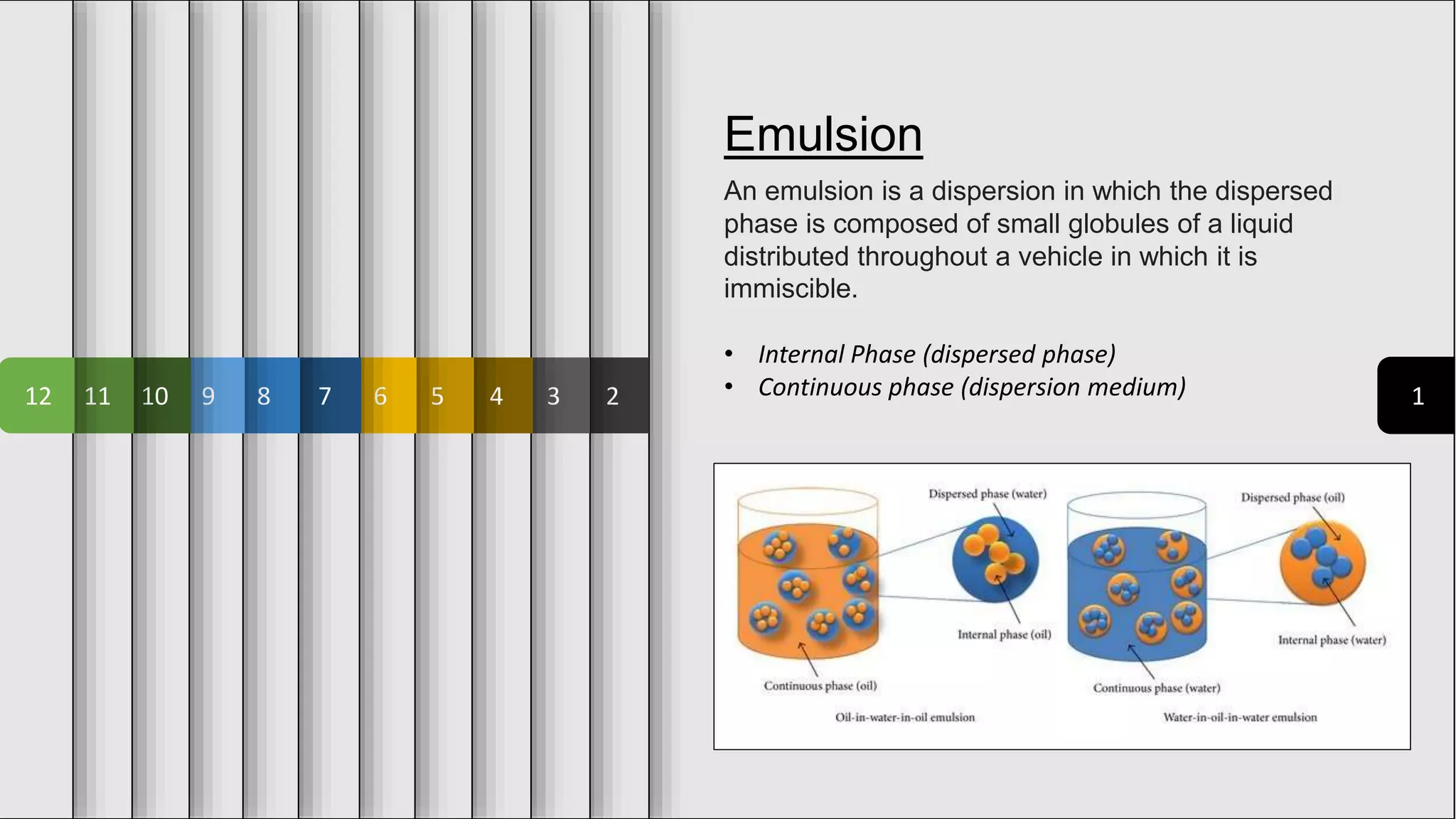
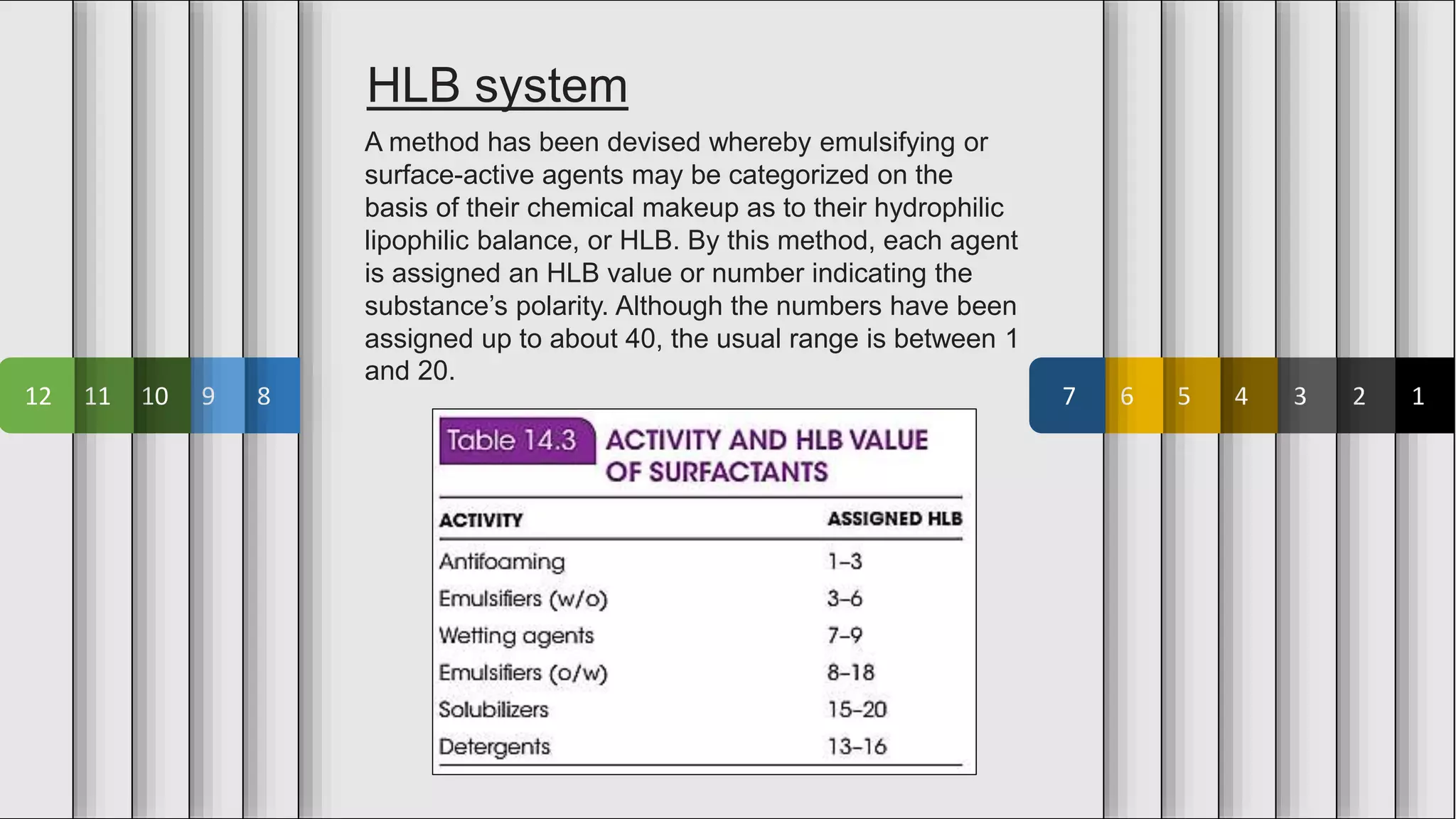
The document provides an overview of emulsions in pharmaceutical dosage forms, including definitions, purposes, and theories of emulsification. It describes emulsifying agents, the HLB system for categorizing agents based on polarity, and various methods of preparation, such as the continental and bottle methods. Additionally, it distinguishes between microemulsions and macroemulsions based on droplet size and stability.