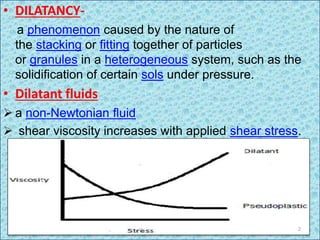
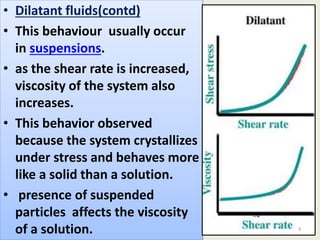
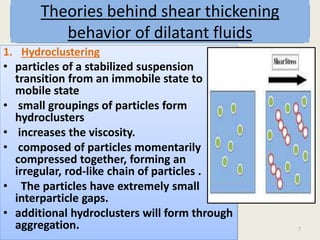
Dilatant fluids, or shear thickening fluids, exhibit increased viscosity with applied shear stress, behaving more like solids under pressure due to particle interactions in heterogeneous systems. This phenomenon is observed in colloidal suspensions, influenced by the ratio of interparticle forces, leading to hydroclustering and order-disorder transitions in particle arrangements. Examples include mixtures like cornstarch and water, as well as non-food substances such as wet sand and slime.