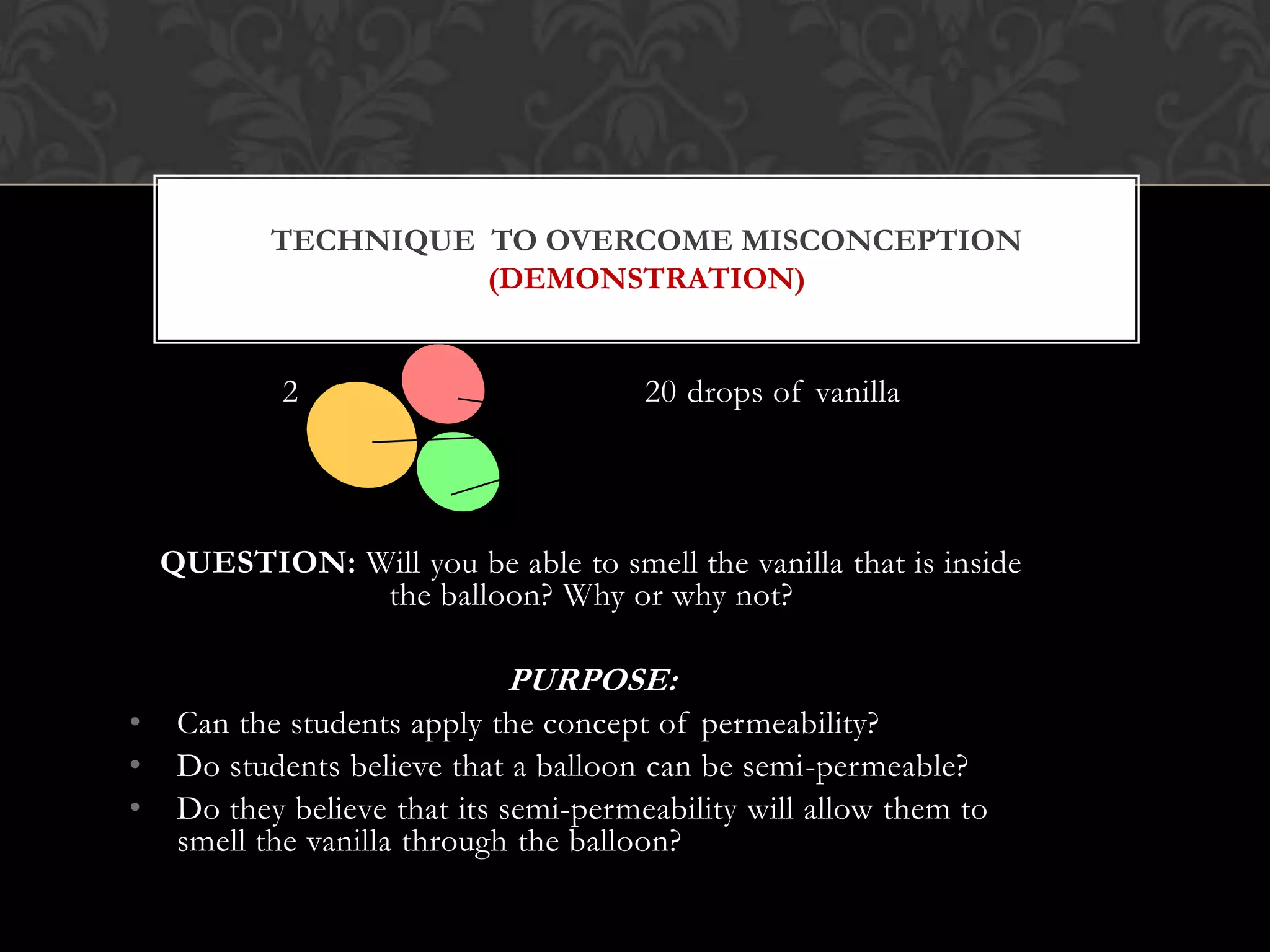
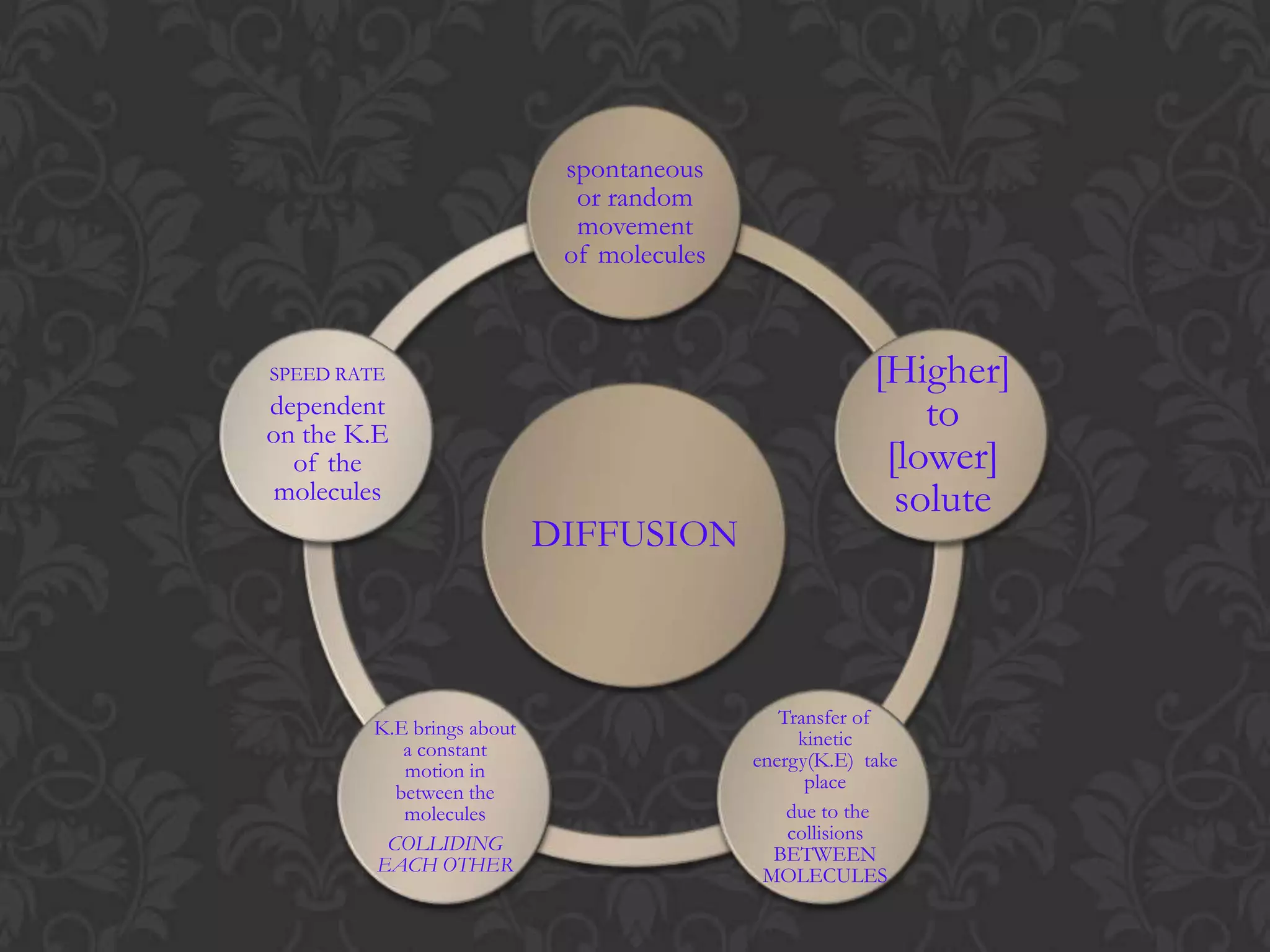
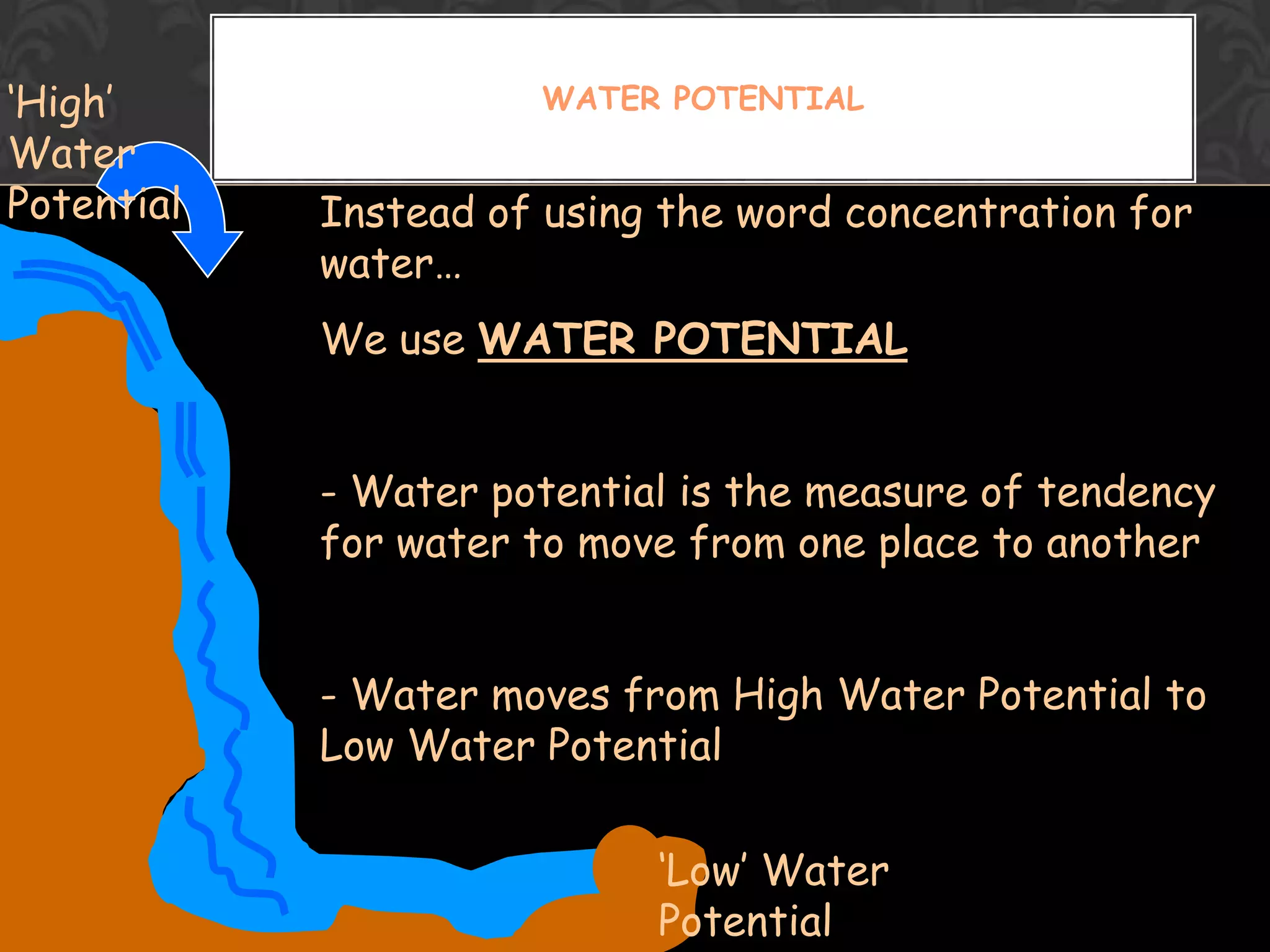
This document discusses the differences and similarities between diffusion and osmosis. Diffusion involves the net movement of particles from an area of higher concentration to lower concentration without a membrane. Osmosis requires a semi-permeable membrane and involves the net movement of water molecules from an area of higher water potential to lower water potential. Both processes aim to evenly distribute particles or solvent, but diffusion can occur over longer distances while osmosis is limited by the membrane. Common misconceptions about these processes are also addressed.




![Flow from [higher] to [lower] of solute](https://image.slidesharecdn.com/diffusionict-101005200352-phpapp02/75/Diffusion-ict-8-2048.jpg)
![Flow from [lower] to [higher] of solvent](https://image.slidesharecdn.com/diffusionict-101005200352-phpapp02/75/Diffusion-ict-9-2048.jpg)

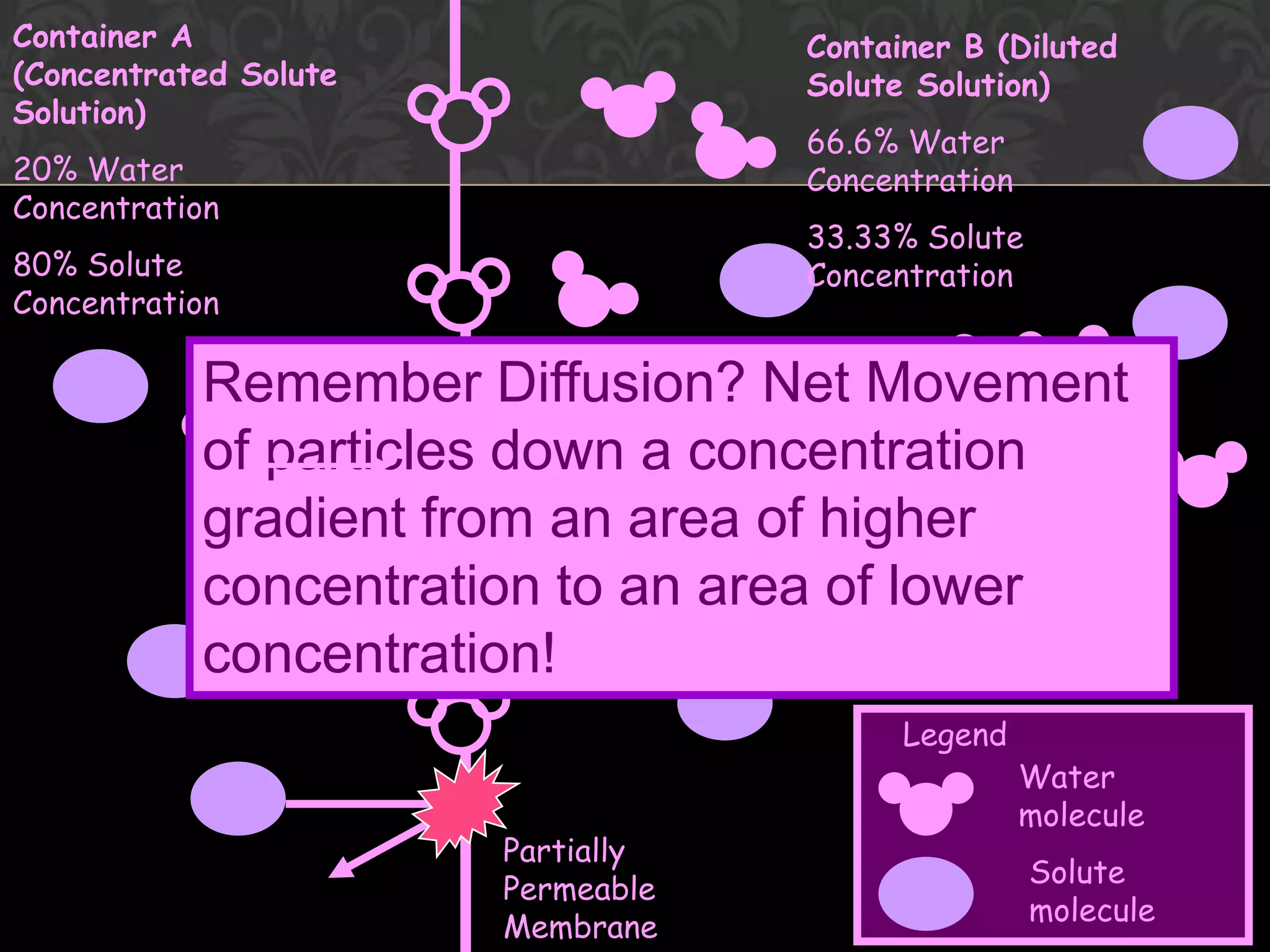

![Flow from [lower] to [higher] of solute](https://image.slidesharecdn.com/diffusionict-101005200352-phpapp02/75/Diffusion-ict-14-2048.jpg)
![Flow from [higher] to [lower] of solvent](https://image.slidesharecdn.com/diffusionict-101005200352-phpapp02/75/Diffusion-ict-15-2048.jpg)

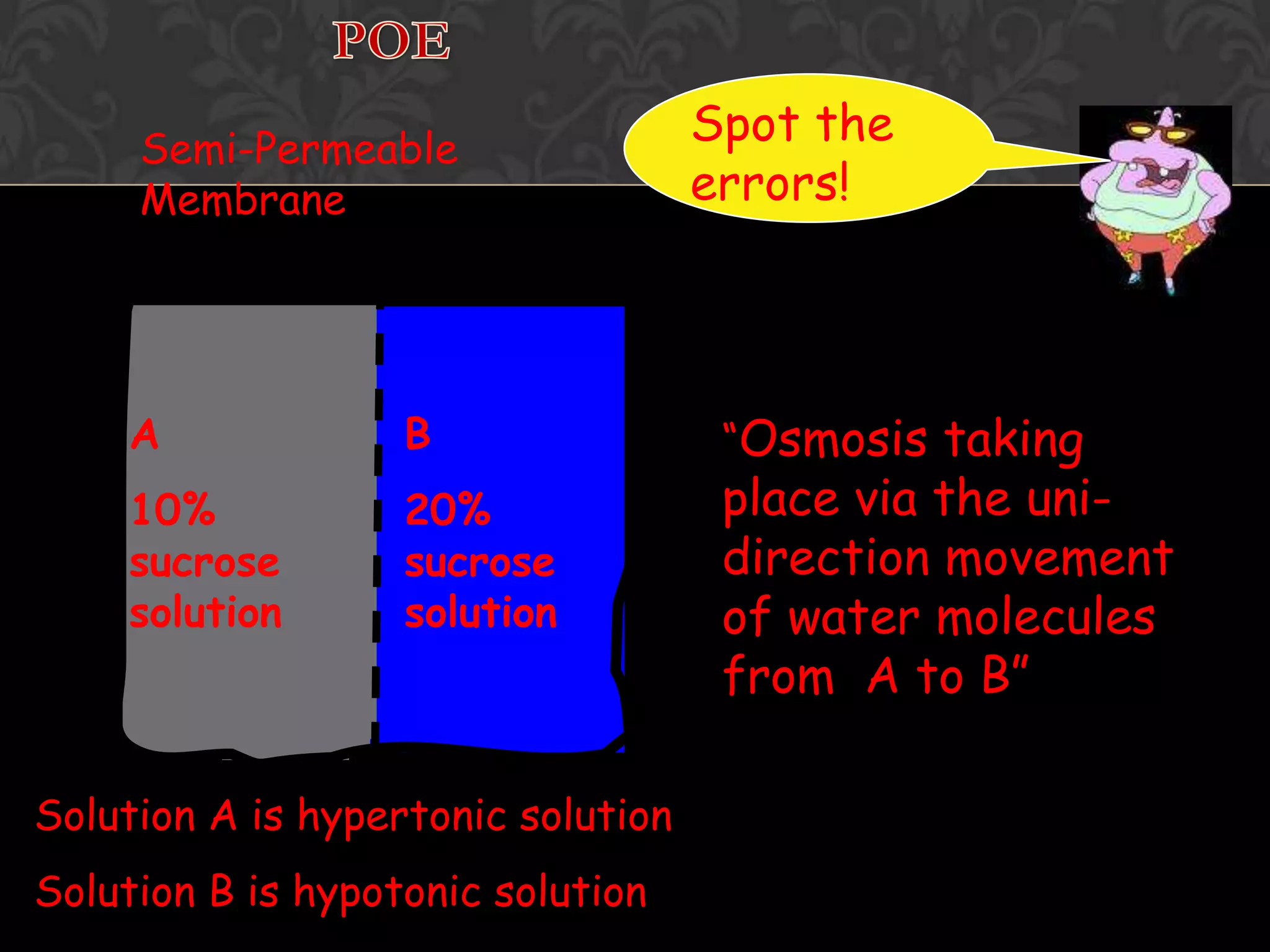
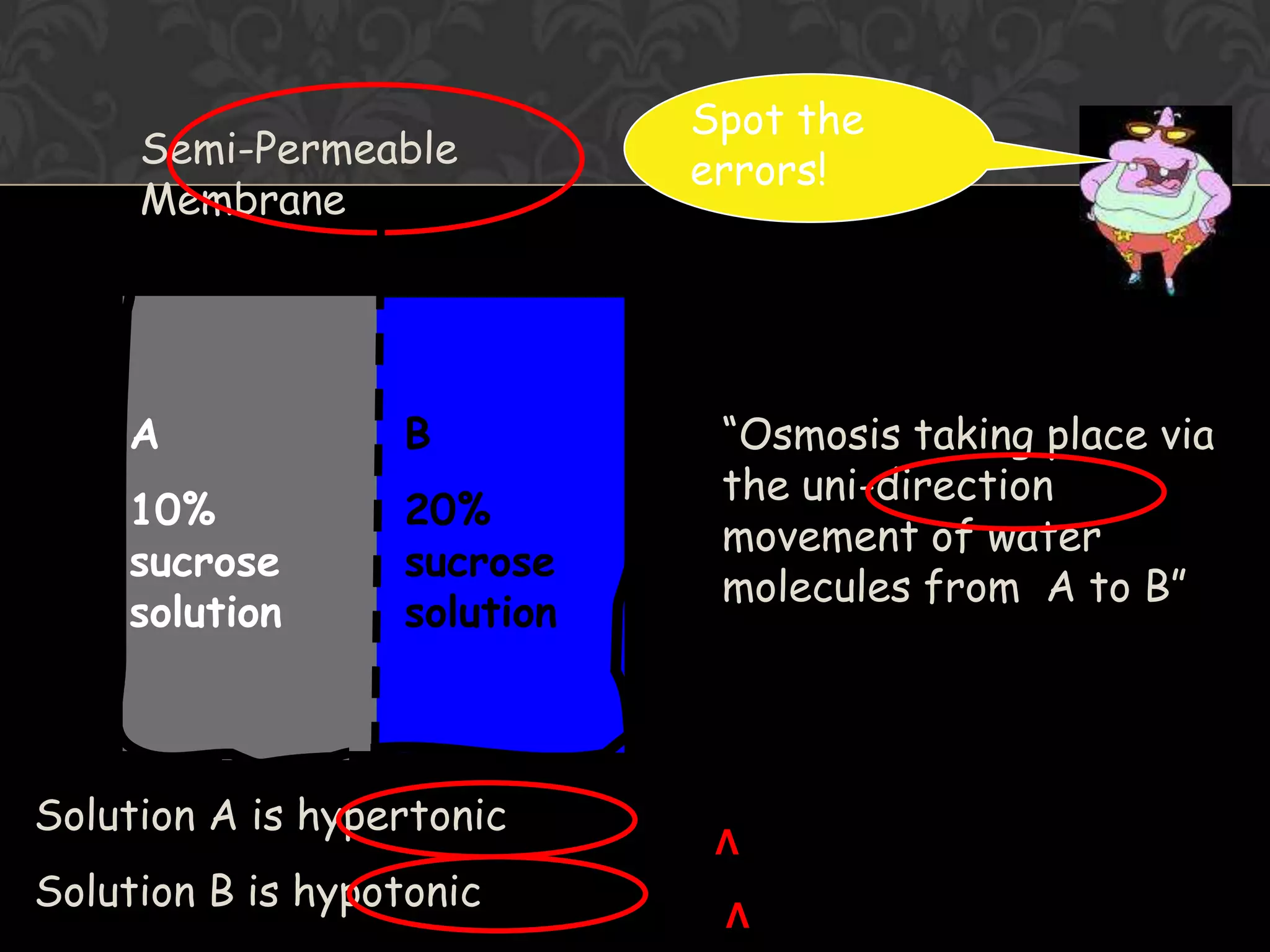
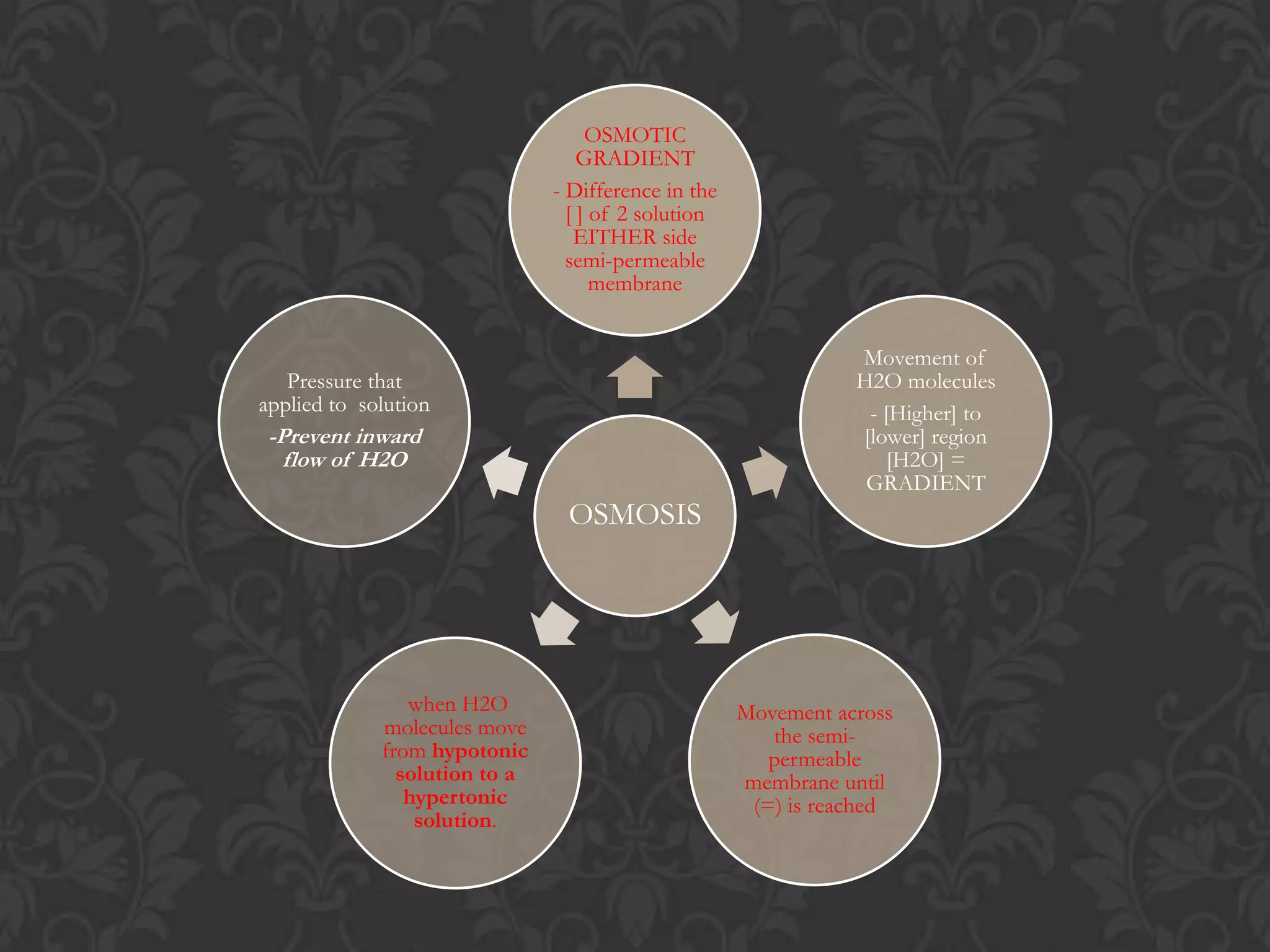
![Osmosis Concept1. Water molecules are always moving about2. Net movement of WATER MOLECULES (Solvent) from a solution of [higher] of water to one with [lower] of water3. Movement of WATER MOLCULES must be through a PARTIALLY PERMEABLE MEMBRANE](https://image.slidesharecdn.com/diffusionict-101005200352-phpapp02/75/Diffusion-ict-19-2048.jpg)