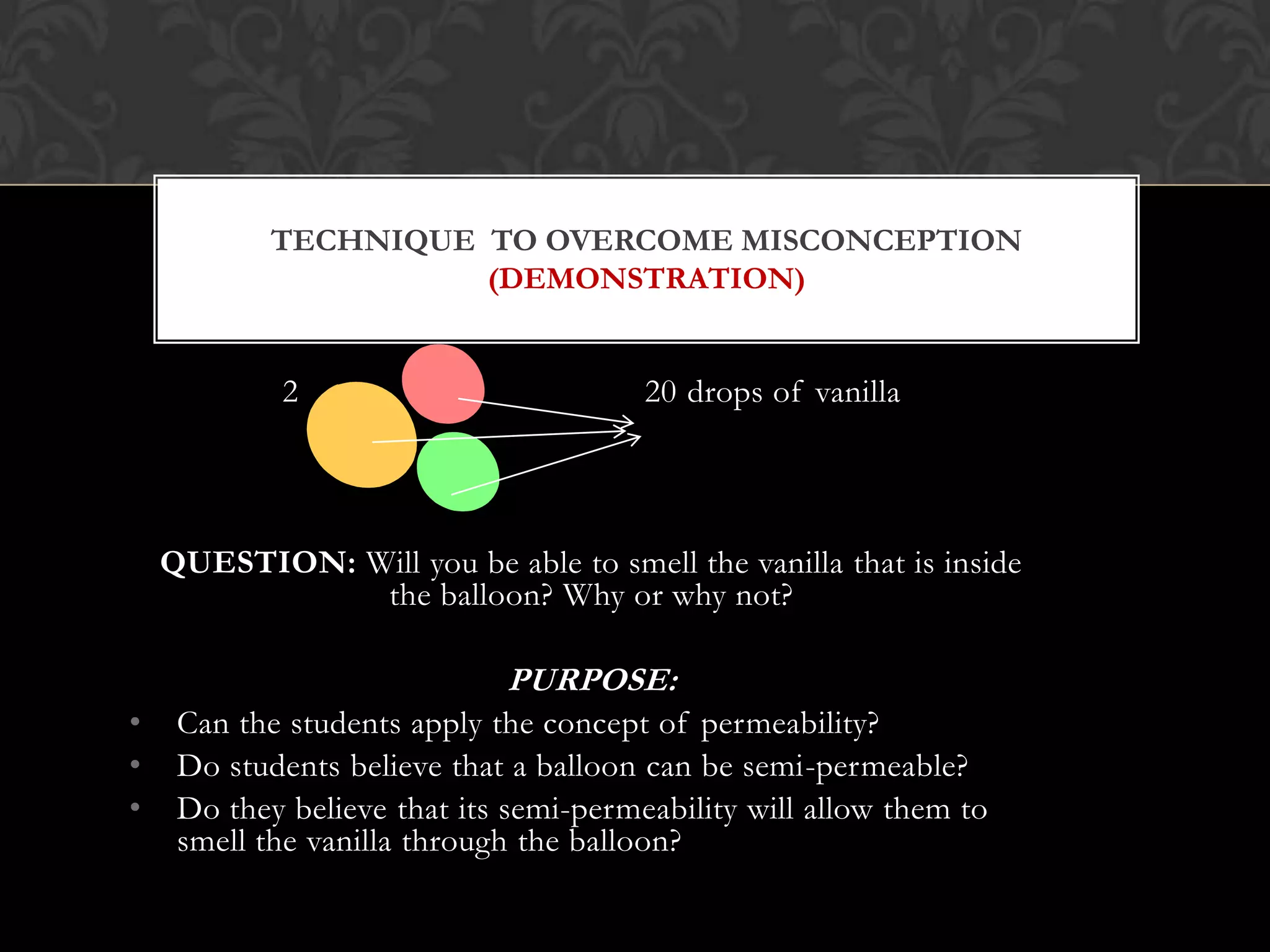
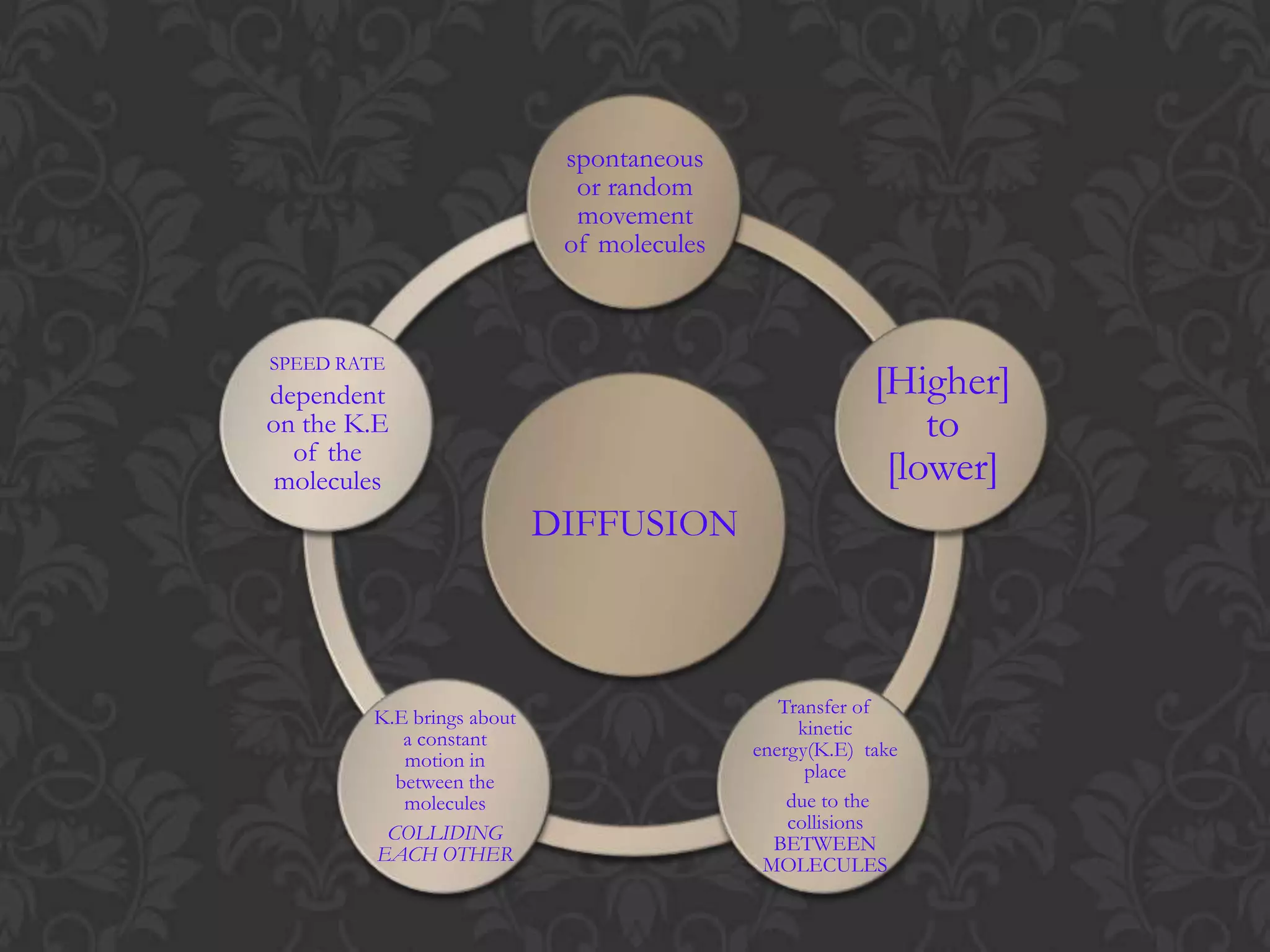
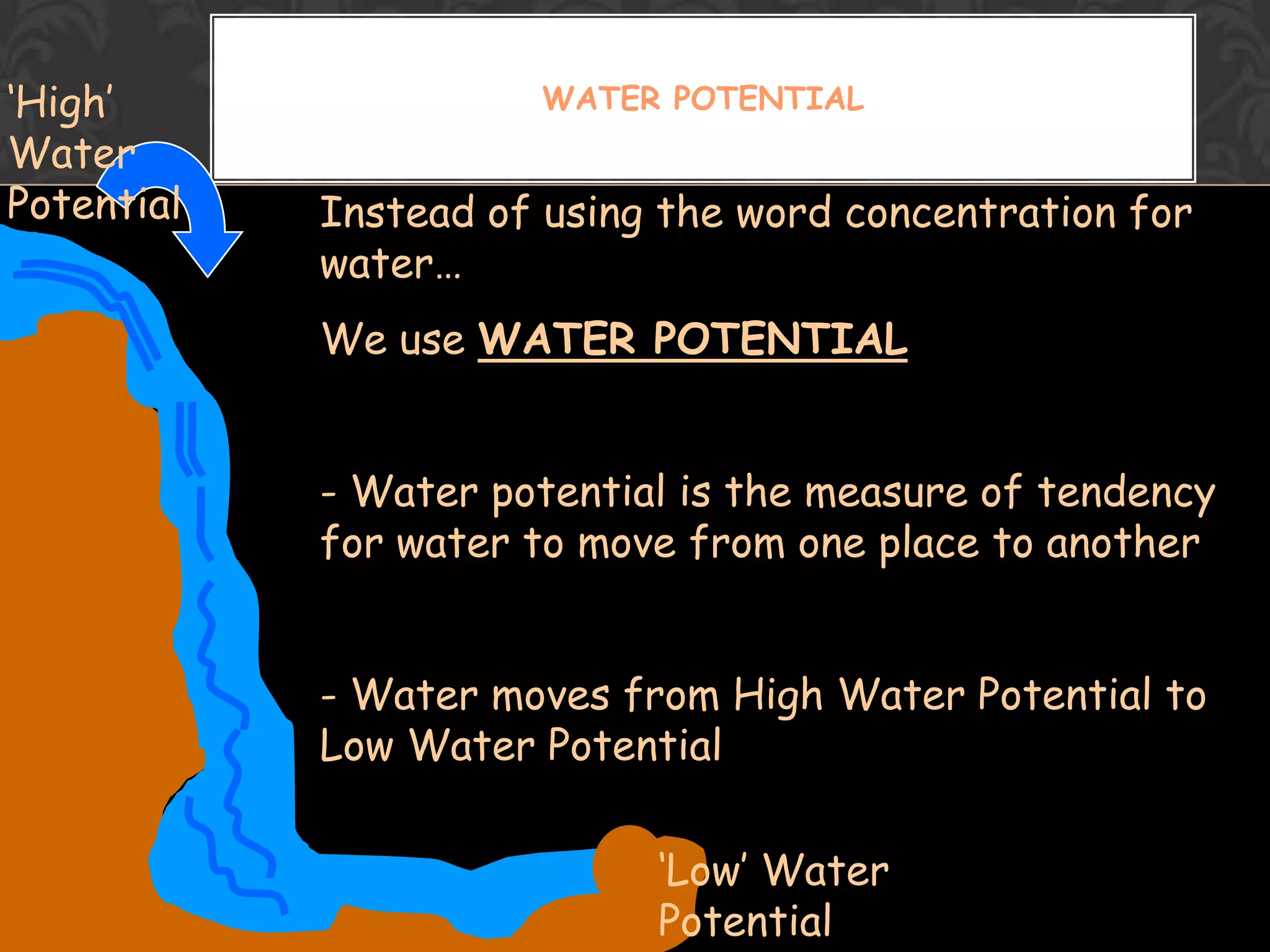
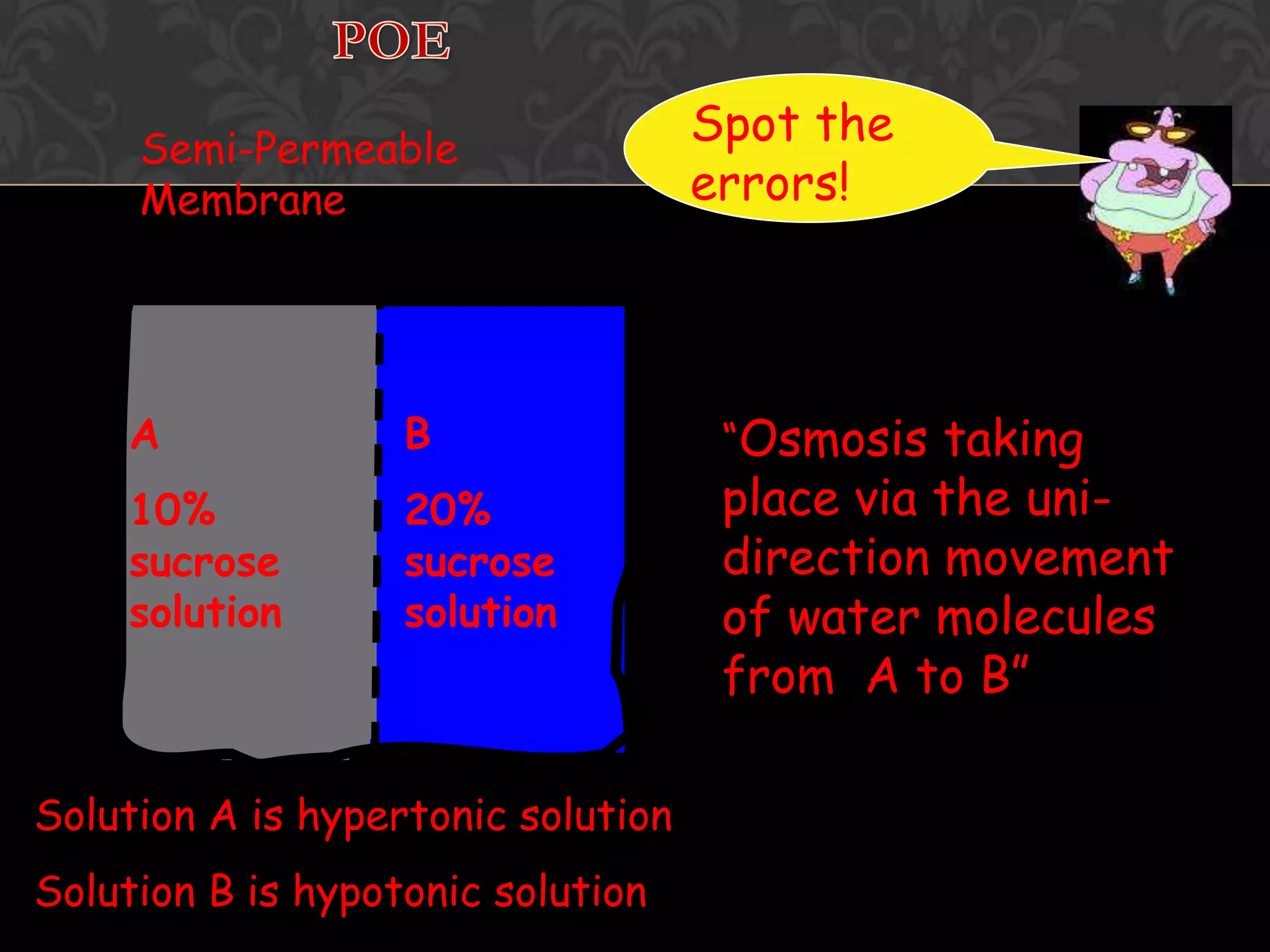
This document discusses the differences and similarities between diffusion and osmosis. It begins by introducing the group members working on the topic. Then, it provides examples and comparisons of diffusion and osmosis. The main differences are that diffusion involves transport across no membrane, while osmosis requires a semi-permeable membrane. Osmosis also involves the movement of water, while diffusion can involve any molecules or ions. Their similarities are that both involve the passive transport of substances from an area of higher concentration to lower concentration. The document then discusses misconceptions students may have and provides activities to help overcome them, such as using demonstrations involving semi-permeable membranes.




![Flow from [higher] to [lower] of solute](https://image.slidesharecdn.com/diffusionict-101005200300-phpapp01/75/Diffusion-Vs-Osmosis-10-2048.jpg)
![Flow from [lower] to [higher] of solvent](https://image.slidesharecdn.com/diffusionict-101005200300-phpapp01/75/Diffusion-Vs-Osmosis-11-2048.jpg)

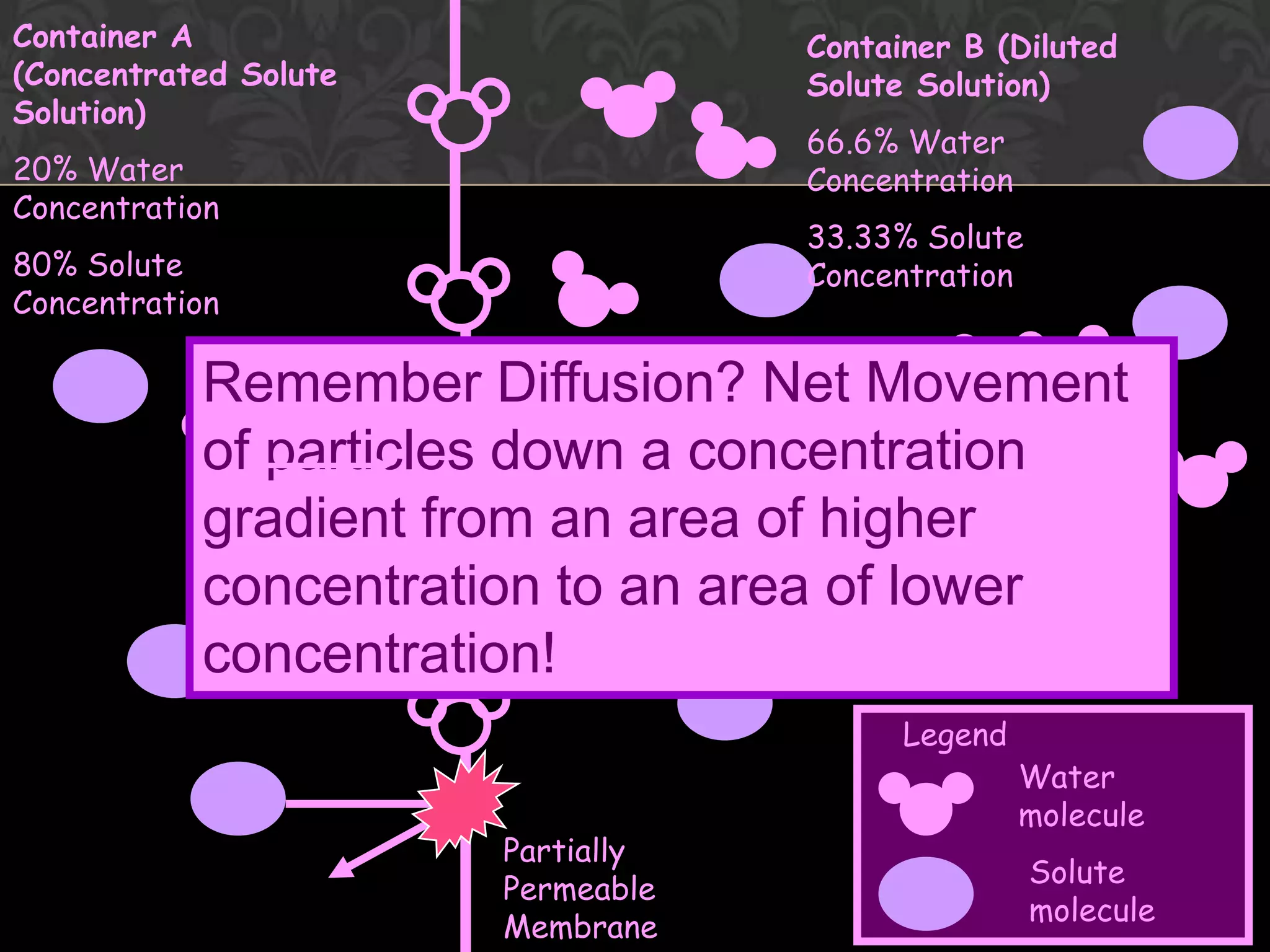

![Flow from [lower] to [higher] of solute](https://image.slidesharecdn.com/diffusionict-101005200300-phpapp01/75/Diffusion-Vs-Osmosis-16-2048.jpg)
![Flow from [higher] to [lower] of solvent](https://image.slidesharecdn.com/diffusionict-101005200300-phpapp01/75/Diffusion-Vs-Osmosis-17-2048.jpg)

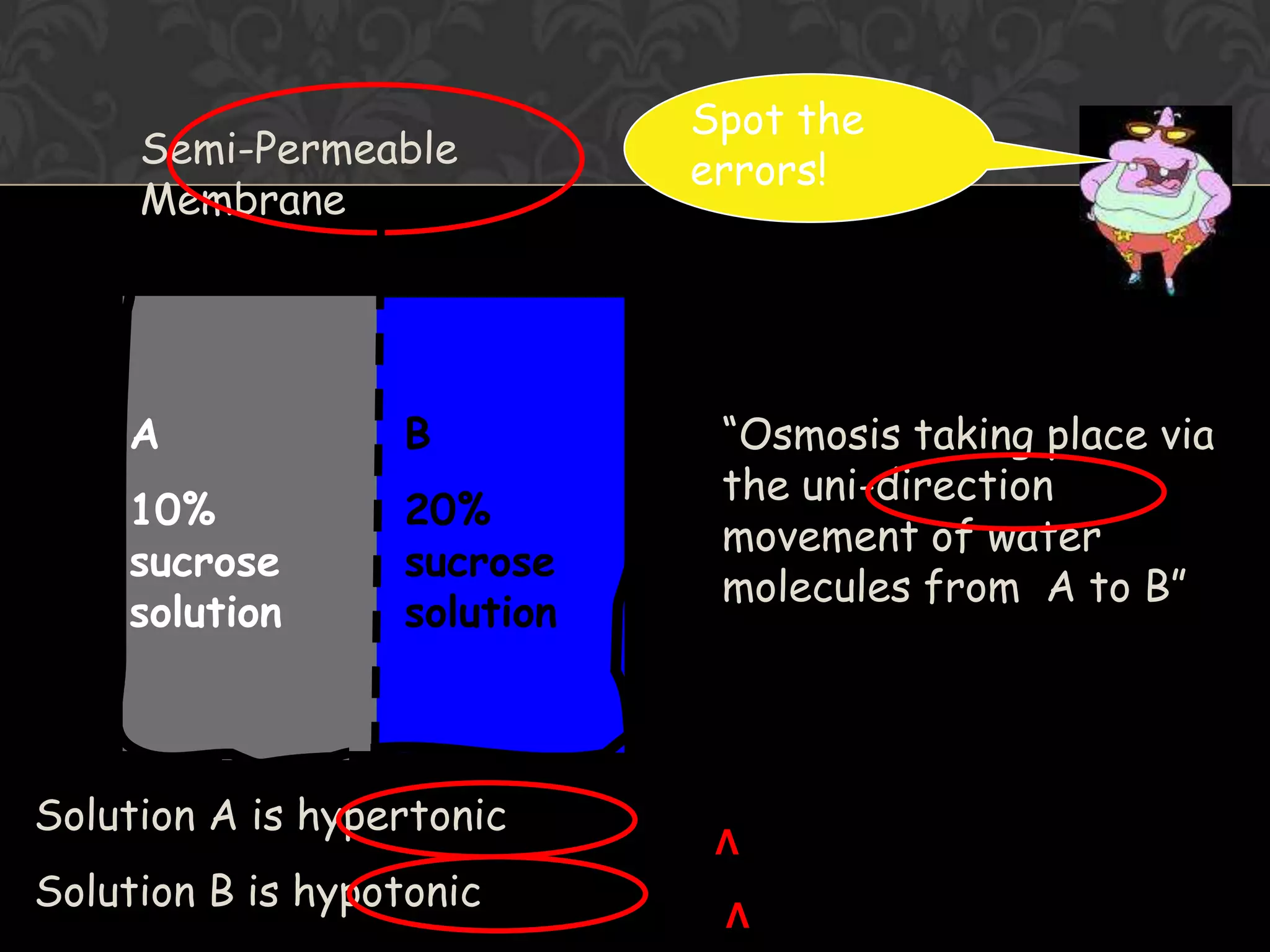
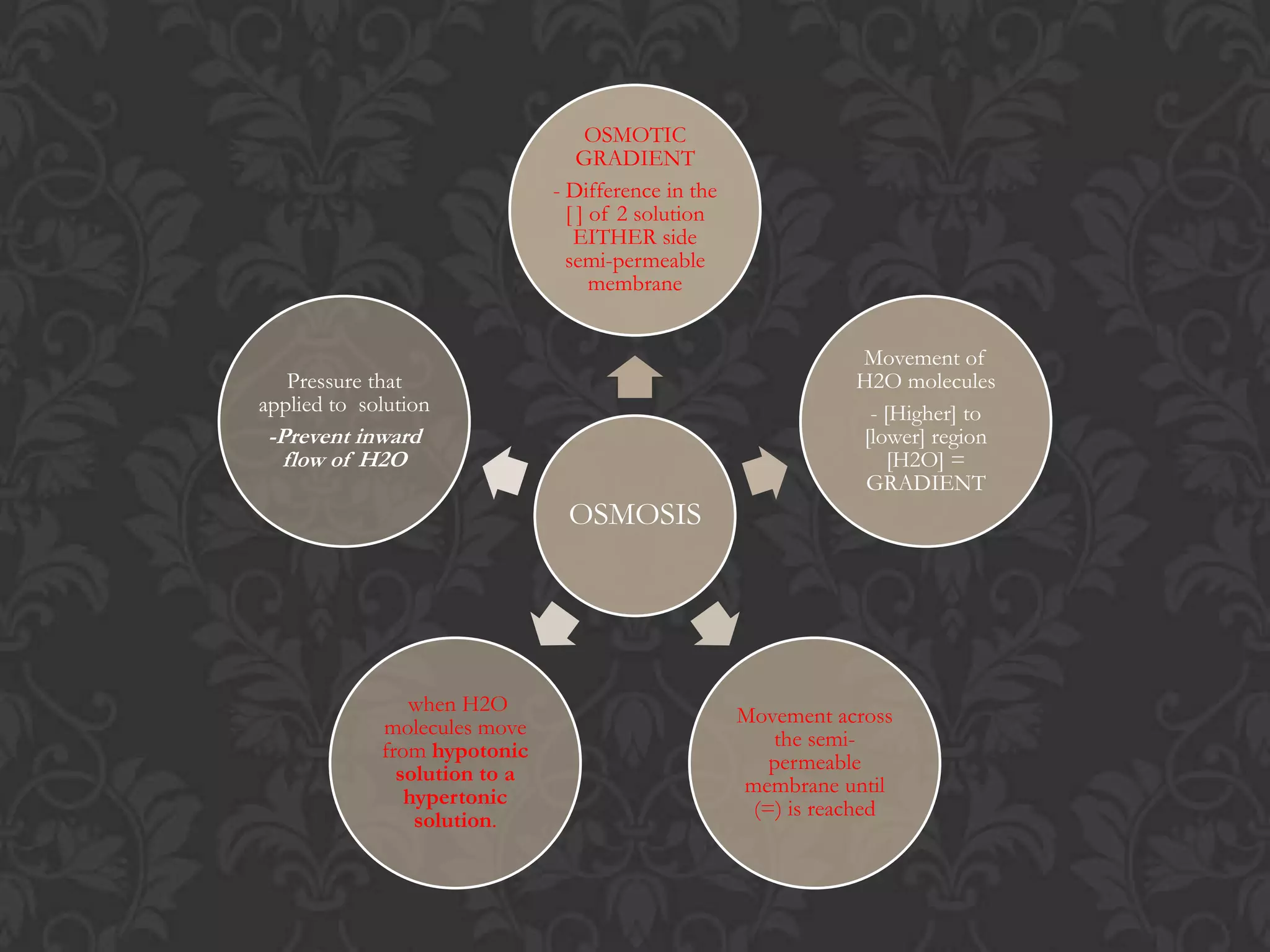
![Osmosis Concept1. Water molecules are always moving about2. Net movement of WATER MOLECULES (Solvent) from a solution of [higher] of water to one with [lower] of water3. Movement of WATER MOLCULES must be through a PARTIALLY PERMEABLE MEMBRANE](https://image.slidesharecdn.com/diffusionict-101005200300-phpapp01/75/Diffusion-Vs-Osmosis-21-2048.jpg)