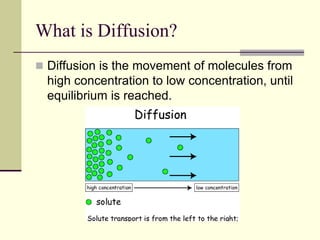
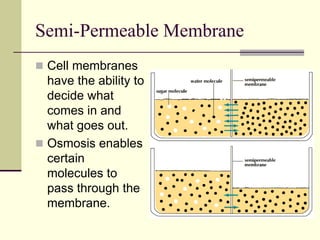
This document discusses diffusion and osmosis in cells. Diffusion is the movement of molecules from areas of high concentration to low concentration until equilibrium is reached. Equilibrium occurs when there is no net movement of particles across a space or boundary. Osmosis is a type of diffusion that occurs across a semi-permeable membrane, allowing only certain molecules like water to pass through. Osmosis regulates water transport and turgor pressure in cells, maintaining homeostasis. It is an important process that occurs in human cells through diffusion and is utilized in applications like desalination and water purification.