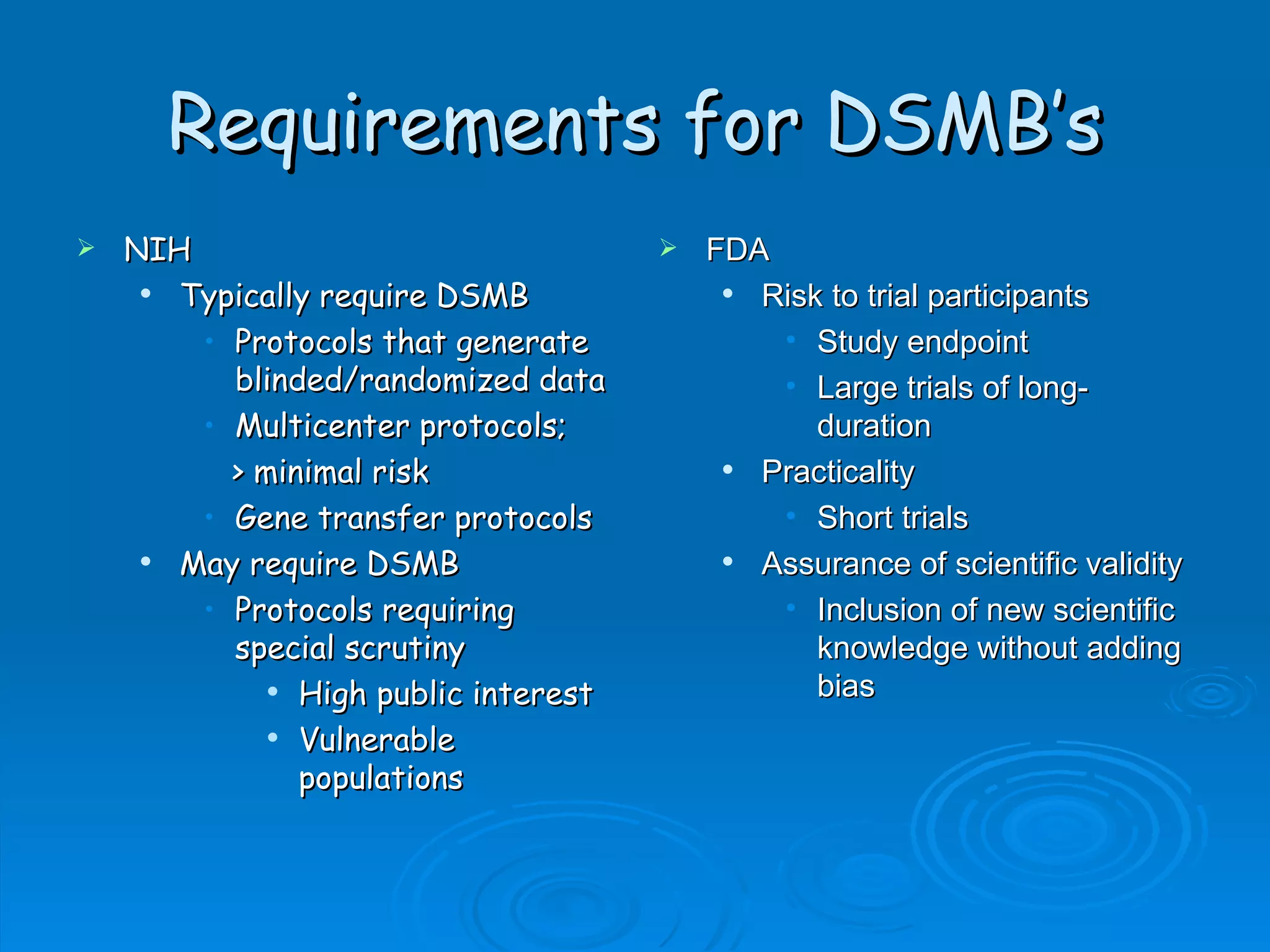
A Data and Safety Monitoring Board (DSMB) is a committee that monitors clinical trials for safety, efficacy, and study progress. A DSMB reviews data at regular intervals to ensure the well-being of trial participants and the validity of the study results. The composition of a DSMB includes experts in relevant medical fields as well as statisticians. A DSMB makes recommendations to investigators and sponsors regarding continuation, modification, or termination of a trial based on their periodic assessments.