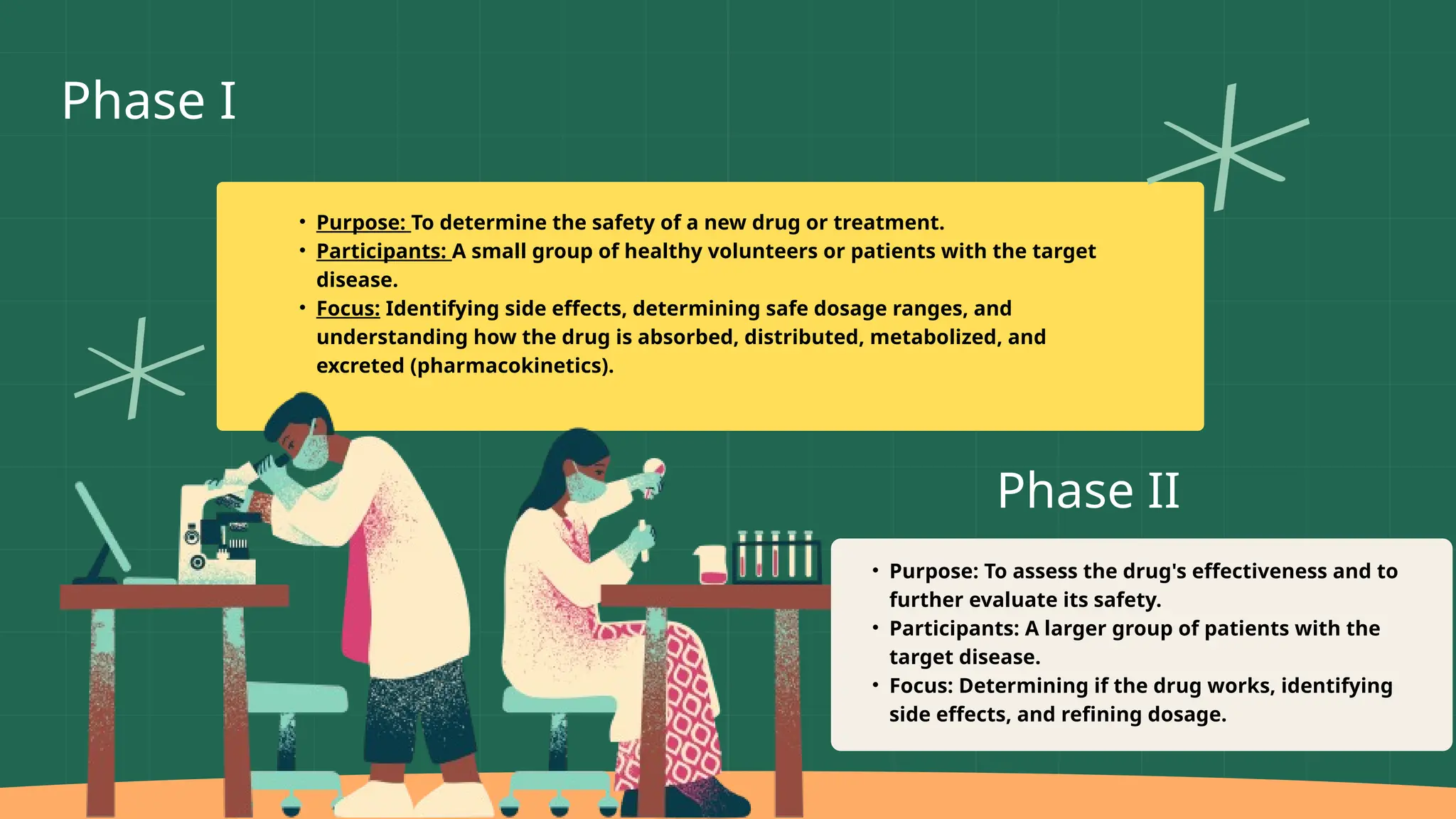
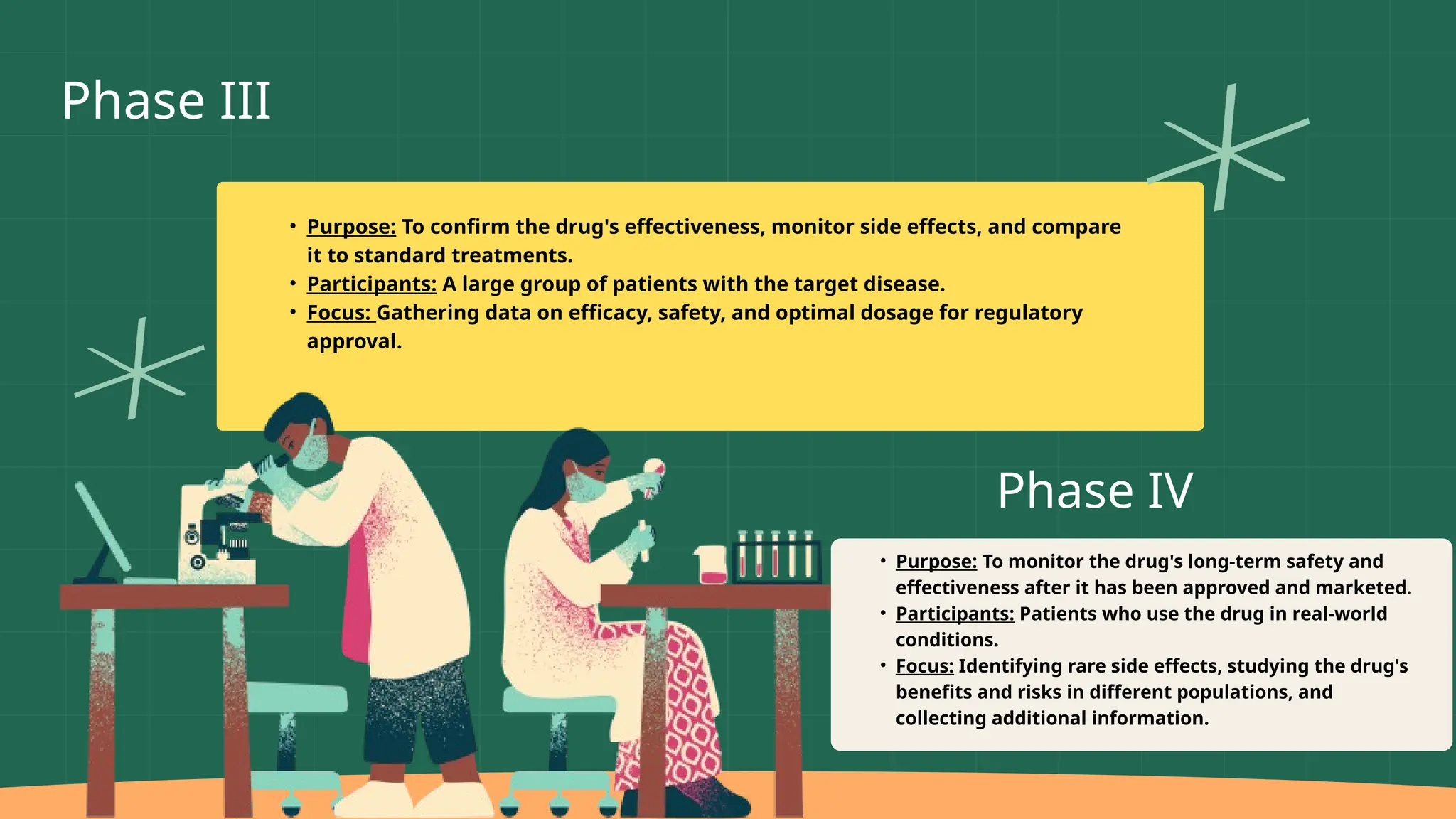
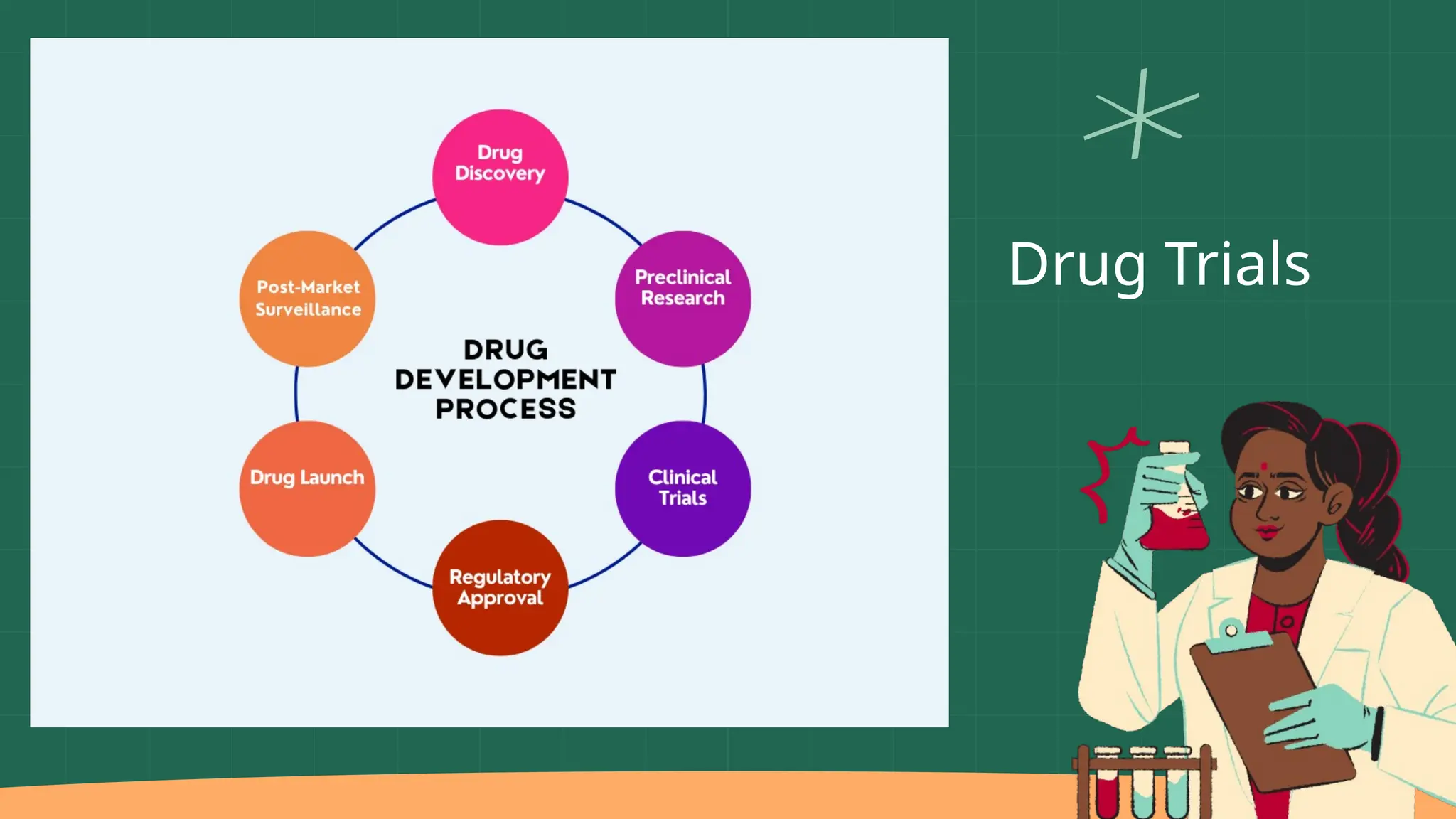
The presentation by Dr. Muhaed Farhaan discusses clinical trials as essential research studies that test new medical interventions, assess their safety and effectiveness, and contribute to public health advancements. It covers the phases of clinical trials, the importance of placebo-controlled trials, and highlights historical ethical breaches in medical research that have led to stricter regulations. Additionally, it explores the complexities and ethical considerations involved in trials for vaccines, medical devices, and organ transplantation.