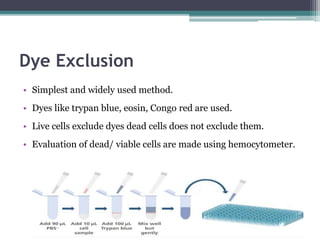
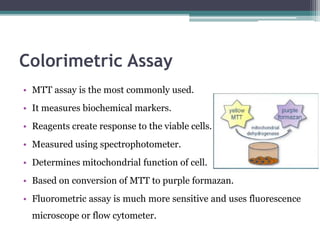
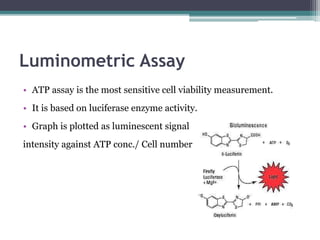
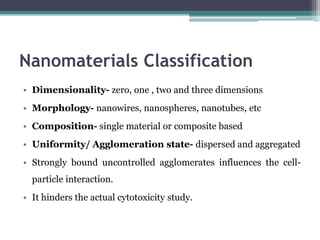
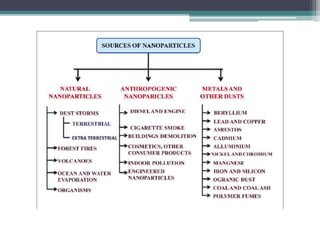
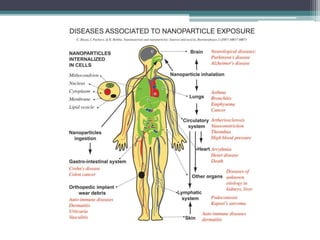
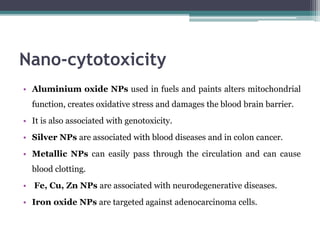
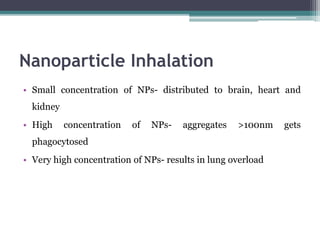
The document discusses cytotoxicity, defined as the ability of drugs and chemicals to destroy living cells, influenced by various stimuli and environmental factors. It covers in-vitro assays for testing cytotoxicity, including endpoint assessments, such as dye exclusion and colorimetric assays, along with the study of nanotoxicology, which examines the toxicity of nanomaterials. It highlights the effects of nanomaterials on cellular functions, potential health risks, and the implications for various diseases.