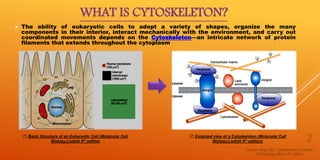
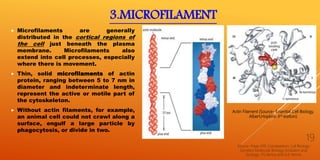
The document discusses the cytoskeleton. It begins by defining the cytoskeleton as an intricate network of protein filaments that extends throughout the cytoplasm and allows eukaryotic cells to maintain their shape, organize their interior components, interact mechanically with the environment, and move in a coordinated fashion.
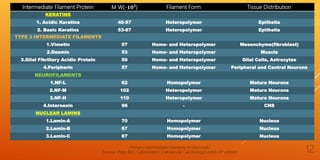
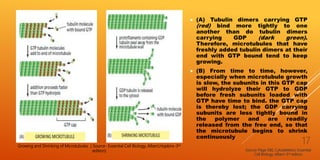
It then describes the three main types of cytoskeletal filaments - intermediate filaments, microtubules, and microfilaments. Intermediate filaments provide tensile strength and anchor cells together. Microtubules act as tracks for intracellular transport and form the mitotic spindle during cell division. Microfilaments are involved in cell motility and contraction.
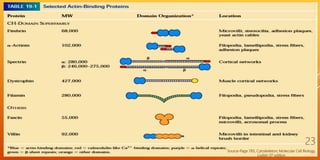
The document goes on to provide more detailed information about each type of