Embed presentation
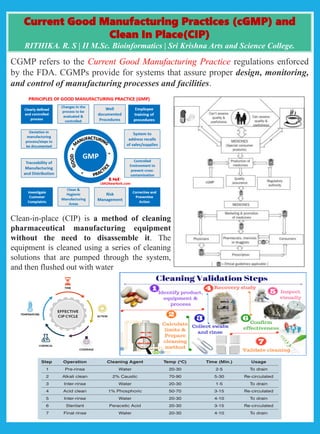
Current Good Manufacturing Practices (CGMP) are FDA regulations that ensure proper design, monitoring, and control of manufacturing processes. Clean-in-Place (CIP) is a method used to clean pharmaceutical equipment without disassembly, utilizing cleaning solutions and flushing with water.
