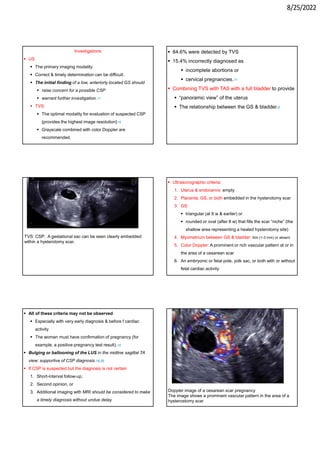
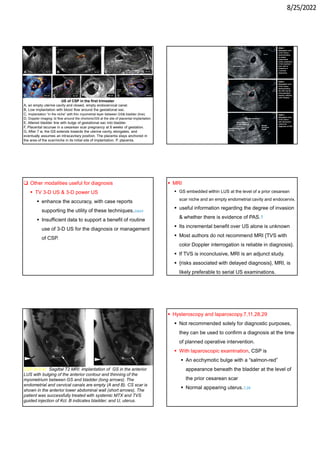
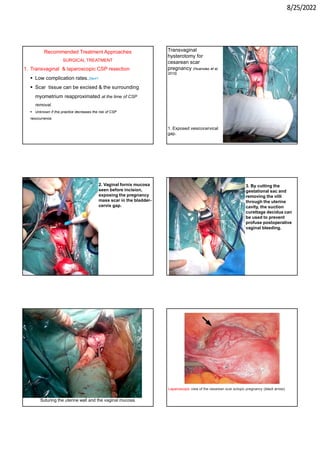
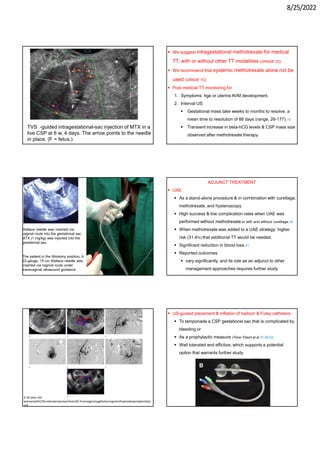
This document provides information about cesarean scar pregnancy (CSP), including its definition, incidence, risk factors, pathogenesis, diagnosis, and treatment. It defines CSP as embryo implantation in the fibrous scar tissue of a prior cesarean hysterotomy. The diagnosis is typically made using transvaginal ultrasound showing gestation within the cesarean scar niche. While expectant management is not recommended due to risks of severe bleeding, treatment options include systemic methotrexate or ultrasound-guided potassium chloride injection to terminate the pregnancy.