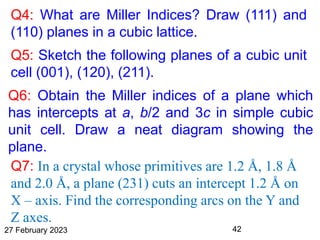
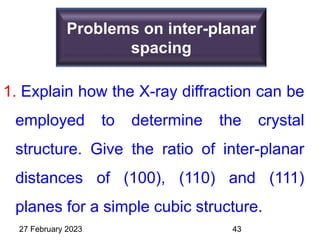
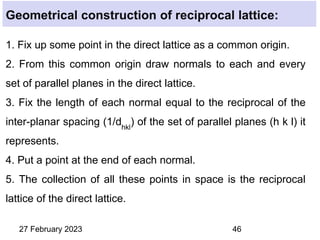
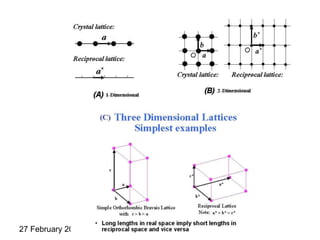
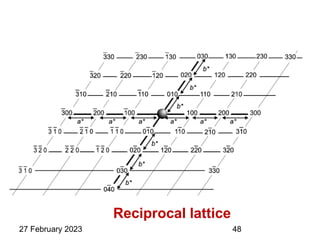
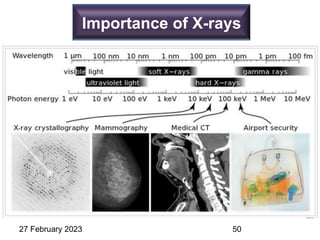
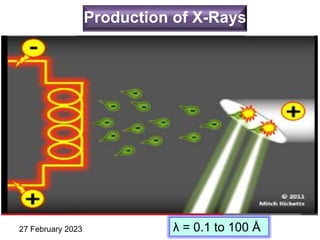
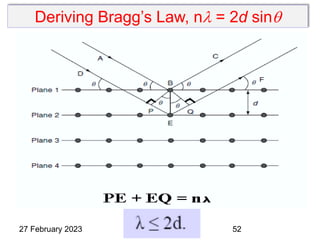
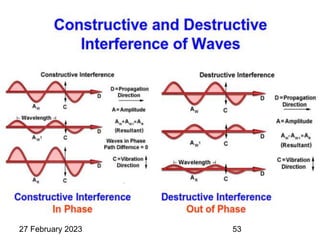
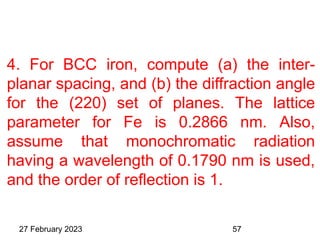
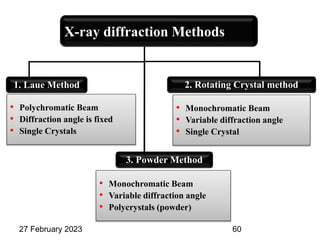
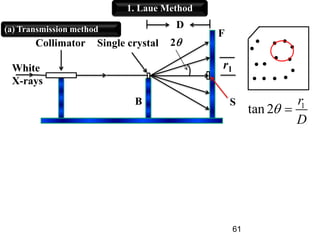
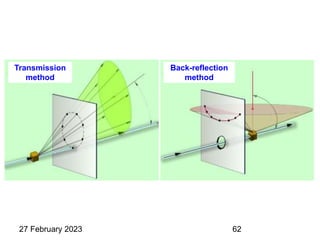
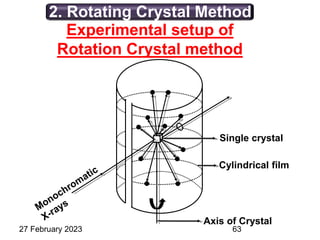
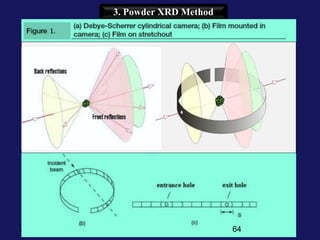
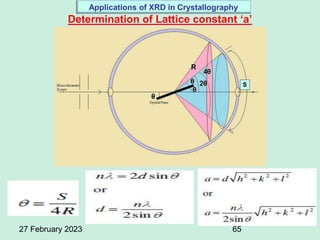
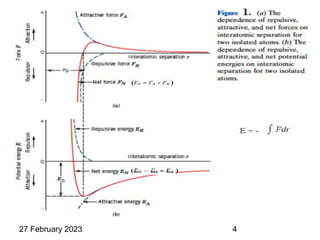
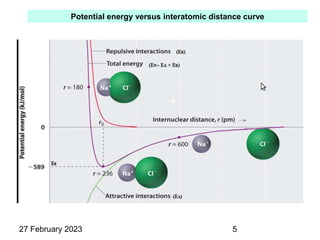
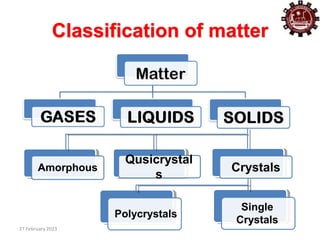
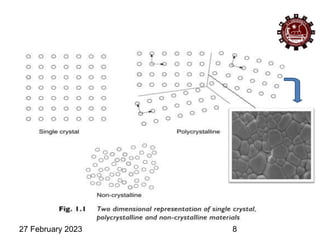
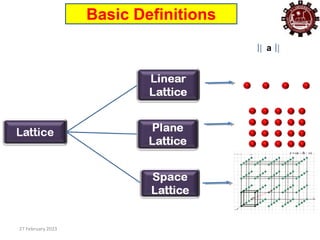
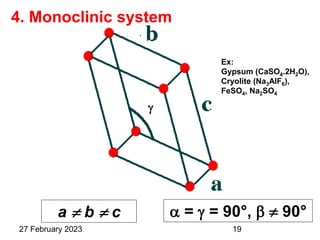
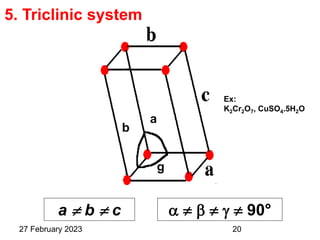
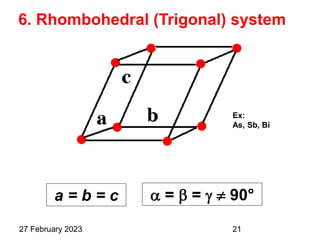
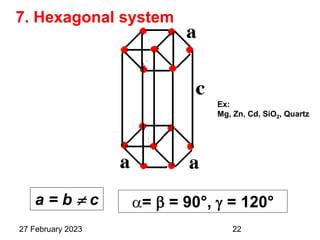
This document provides an overview of crystallography and bonding in solids. It discusses various types of interatomic bonds and classifications of materials, including crystals, quasicrystals, and amorphous solids. It also describes basic crystallographic concepts such as unit cells, Bravais lattices, crystal systems, Miller indices, and X-ray diffraction. Bragg's law for X-ray diffraction is derived. Different X-ray diffraction methods including Laue, rotating crystal, and powder methods are explained. Applications of X-ray diffraction in crystallography are also discussed.








![27 February 2023
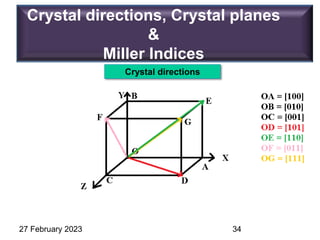
[hkl] Direction, eg. [120]
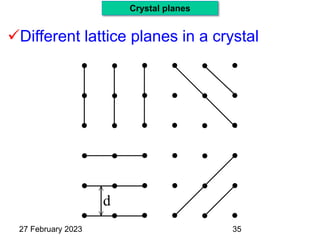
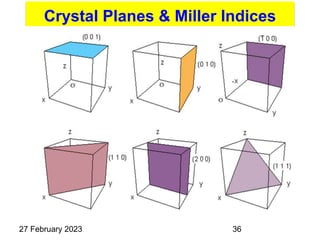
(hkl) Plane, eg. (101)
<hkl> Family of equivalent directions, eg.
{hkl} Family of equivalent planes, eg.
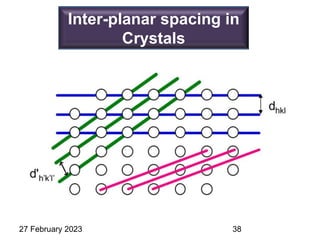
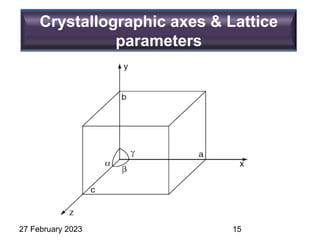
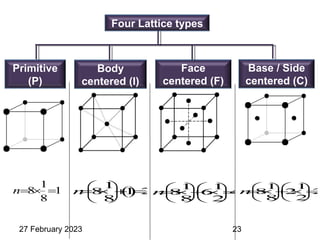
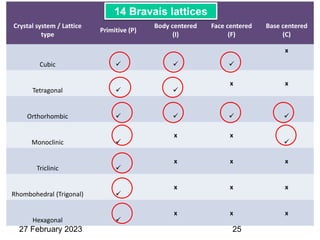
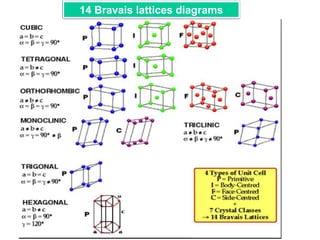
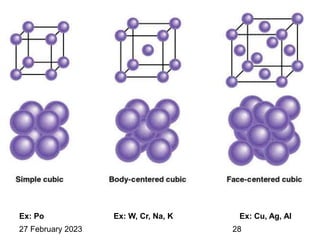
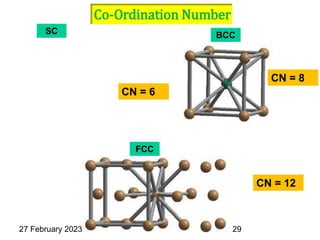
Representation of Crystal directions & Crystal planes
37](https://image.slidesharecdn.com/crystallography-230227044553-0aa25d6c/85/Crystallography-ppt-37-320.jpg)