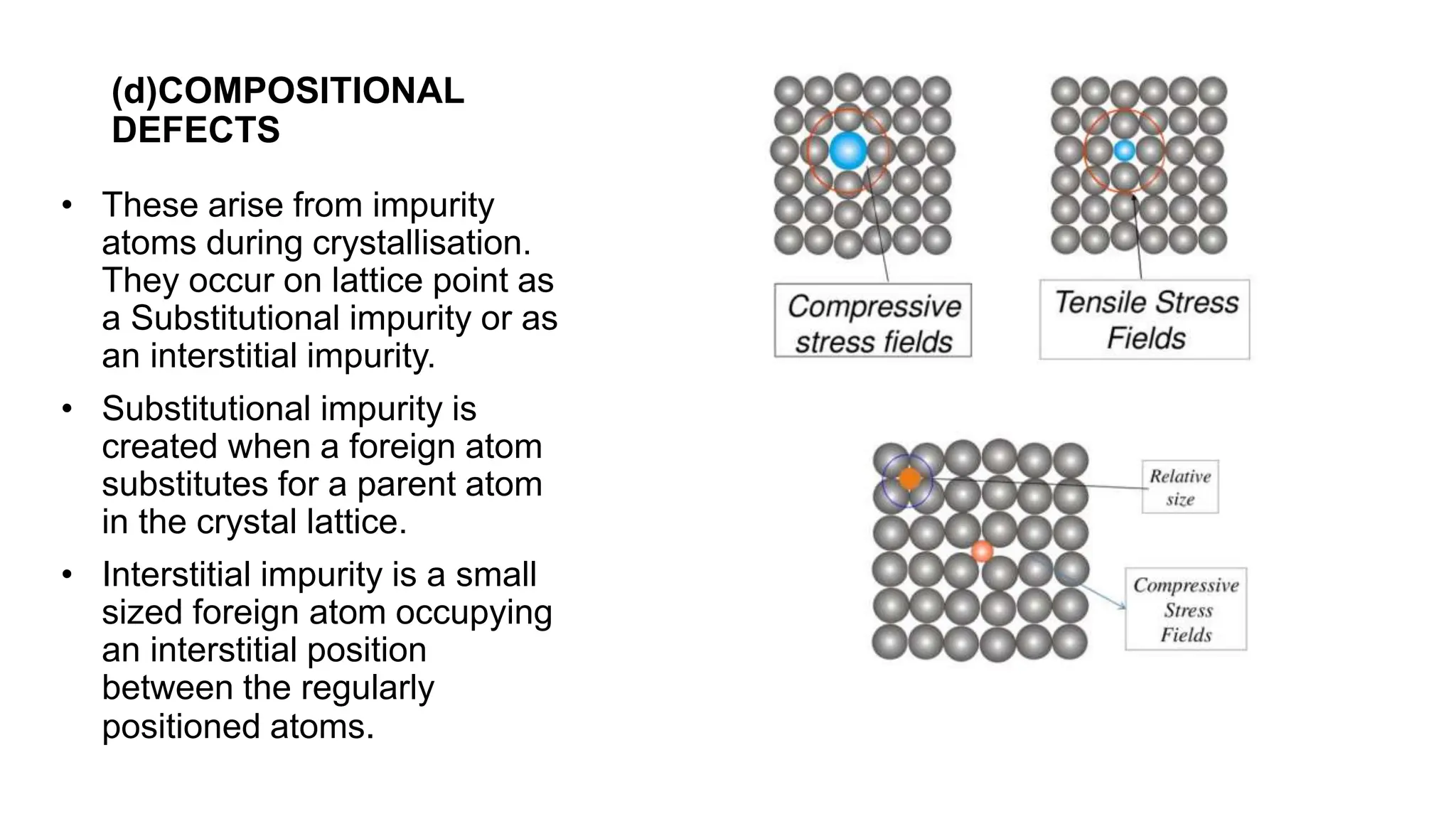
Crystal imperfections refer to defects in the regular geometric arrangement of atoms in a crystal structure. They are inevitable due to factors like rapid cooling, radiation, or thermal vibrations. There are several types of imperfections including point defects, line defects, surface defects, and volume defects. Point defects occur at isolated points and include vacancies, interstitial atoms, and substitutional defects. Line defects called dislocations are one-dimensional defects where atoms are misaligned around a line. Dislocations like edge dislocations are responsible for ductility in materials. Crystal imperfections influence the mechanical, electrical, and optical properties of materials.