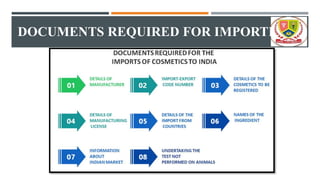
This document summarizes regulatory provisions relating to the import of cosmetics in India. It defines cosmetics according to the Drugs and Cosmetics Act of 1940. It also describes provisions for misbranded, spurious, and adulterated cosmetics. To import cosmetics, compulsory registration with the Drugs Controller General of India is required. The application process takes 6 months and issued certificates are valid for 3 years. Required documents include details of the manufacturer, manufacturing premises, and product details along with a registration fee.