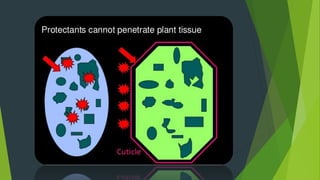
1. The document classifies and describes various types of contact fungicides, including their modes of action. It discusses copper, sulfur, mercury, heterocyclic nitrogen, benzene, quinone, organo tin, organo phosphorus, and miscellaneous contact fungicides.
2. Copper and sulfur fungicides work by disrupting fungal cell metabolism and integrity. Elemental sulfur may produce toxic byproducts like SO2, SO3, or hydrogen sulfide. Organic sulfur fungicides form complexes that reduce fungal growth.
3. Mercury fungicides were highly toxic but are now discouraged due to environmental concerns. Other contact fungicides disrupt fungal enzymes and metabolism through various chemical reactions.