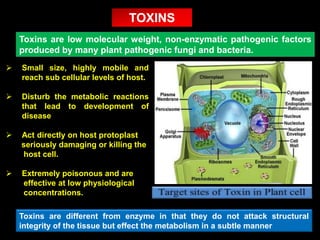
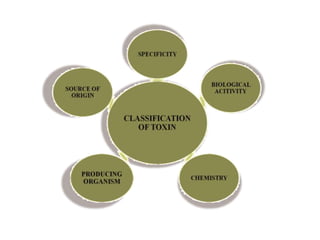
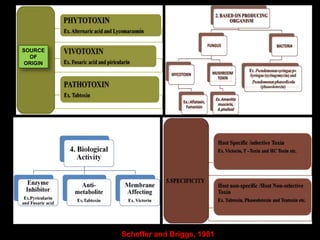
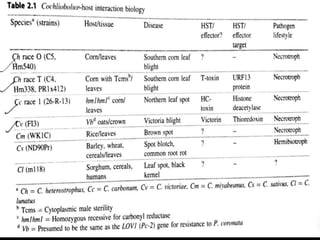
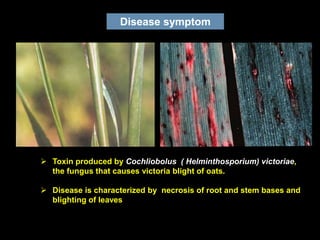
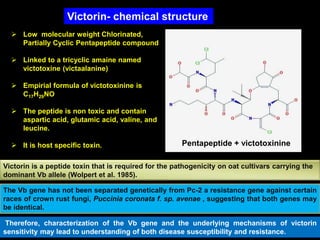
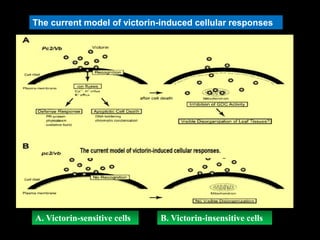
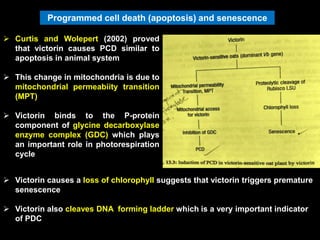
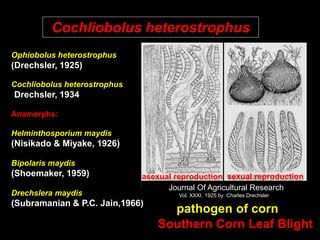
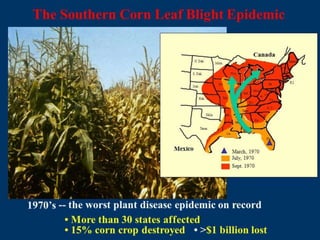
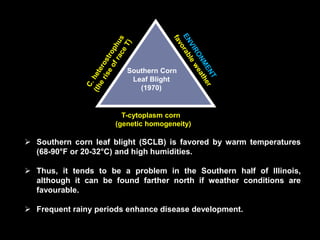
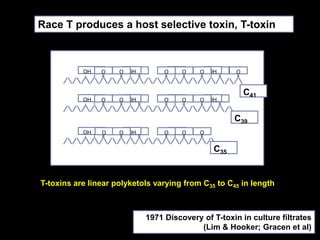
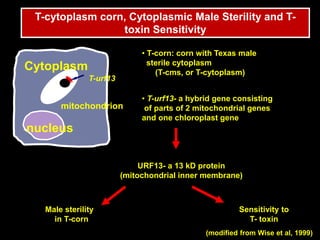
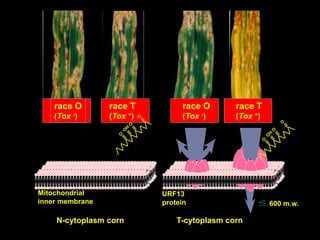
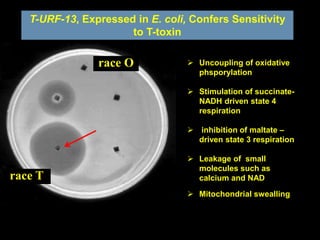
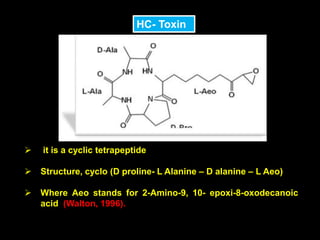
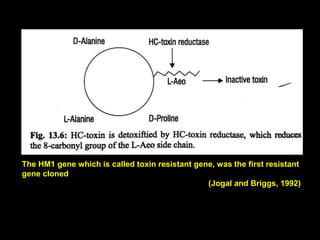
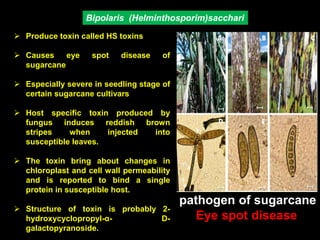
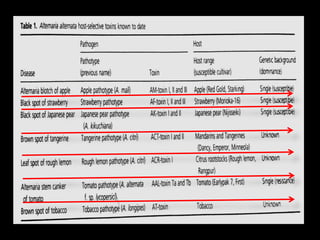
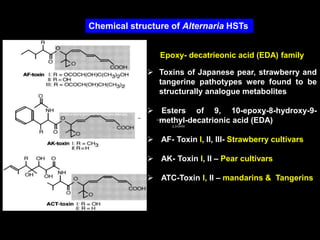
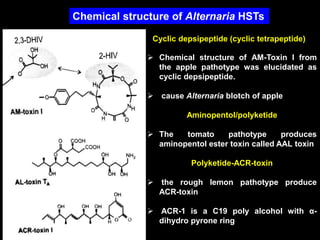
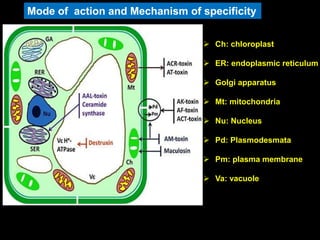
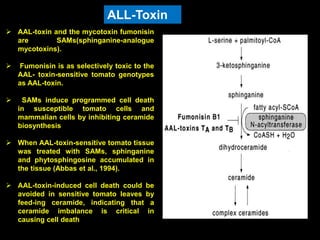
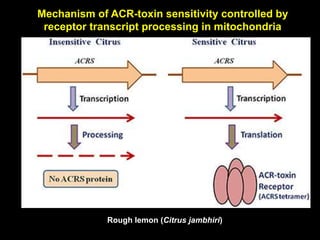
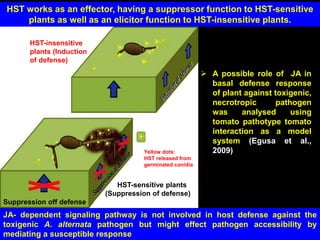
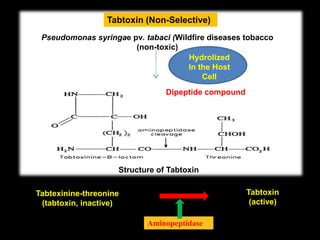
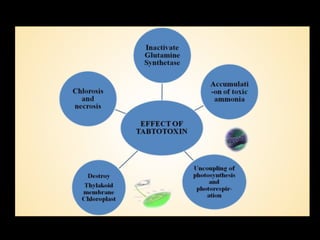
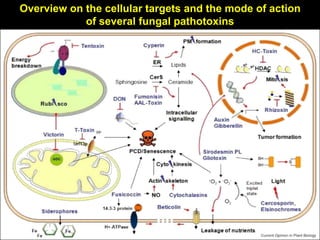
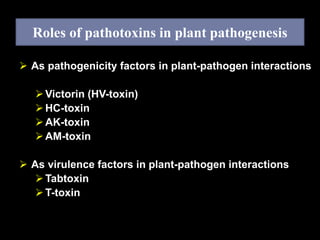
Pathotoxins and plant diseases are discussed. Toxins are low molecular weight compounds produced by plant pathogenic fungi and bacteria that disturb host cell metabolism and cause disease. Notable pathotoxins discussed include victorin from Cochliobolus victoriae which causes oat disease, T-toxin from Bipolaris maydis race T which is host-specific to corn with T-cytoplasm, and HC-toxin from Cochliobolus carbonum race 1 which is also host-specific and causes disease in corn. Alternaria alternata also produces several host-specific toxins on various crops like apple, strawberry, and Japanese pear.