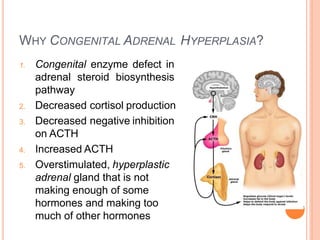
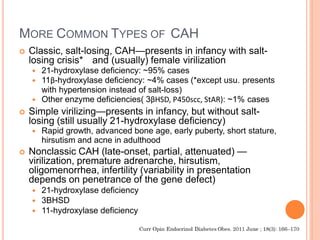
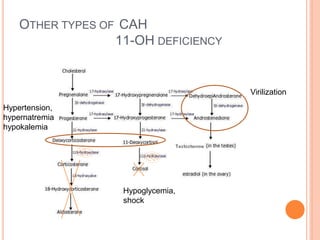
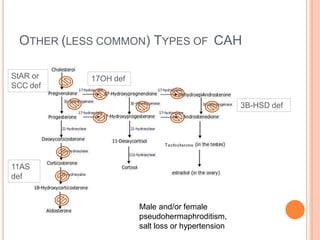
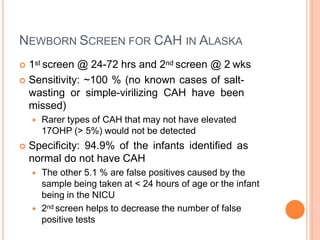
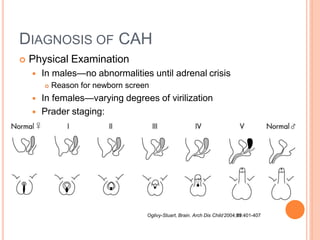
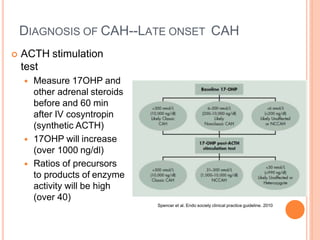
This document discusses congenital adrenal hyperplasia (CAH), which results from a genetic defect in adrenal steroid biosynthesis. It causes decreased cortisol production and increased production of other hormones. The most common type is 21-hydroxylase deficiency, which can present as salt-wasting or simple virilizing CAH. Diagnosis is made through newborn screening, physical exam, and lab tests. Treatment involves hydrocortisone and fludrocortisone supplementation as well as stress dosing during illness to prevent adrenal crisis. Outcomes have improved with newborn screening and lifelong medical management.