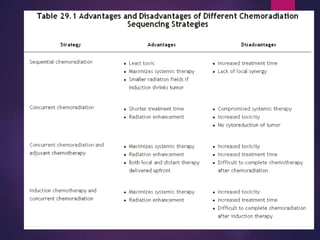
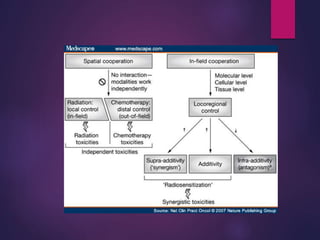
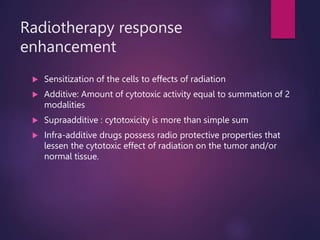
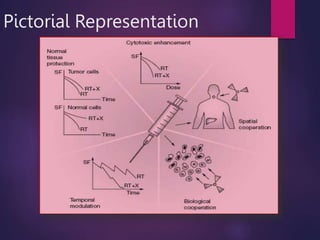
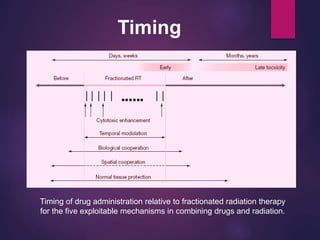
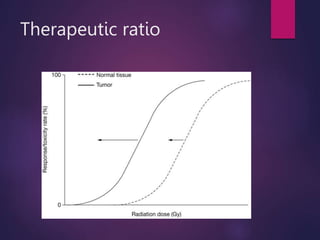
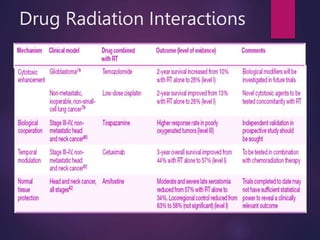
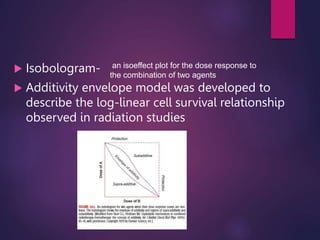
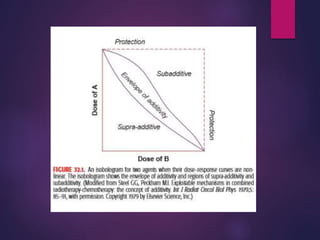
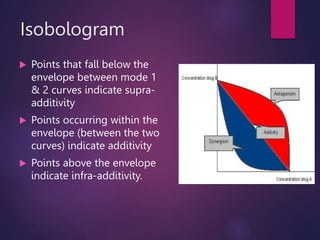
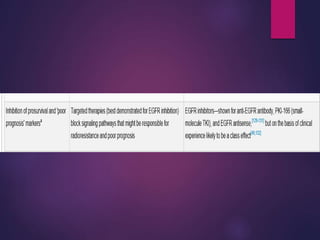
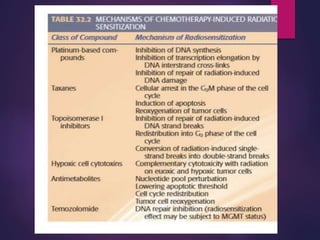
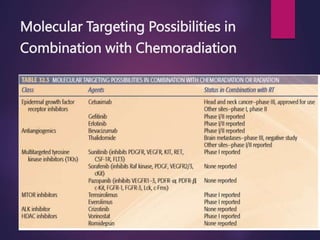
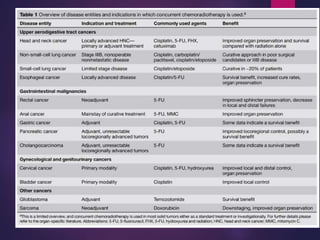
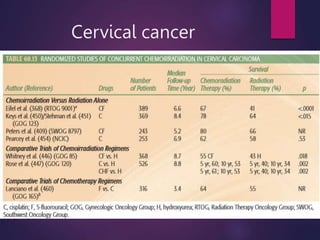
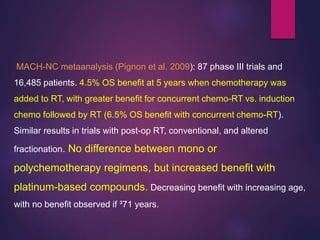
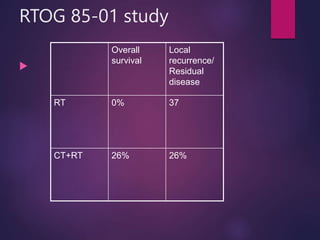
The document discusses the principles and goals of chemoradiotherapy, highlighting the combination of chemotherapy and radiation to improve patient survival and tumor control while minimizing toxicity to normal tissues. It reviews various mechanisms such as spatial cooperation, toxicity independence, and molecular targeting that enhance the effectiveness of treatment, alongside clinical examples from various cancer studies demonstrating the benefits and challenges of this approach. Additionally, it addresses the potential increase in acute toxicities and cost concerns associated with concurrent treatment regimens.