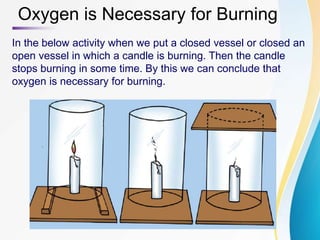
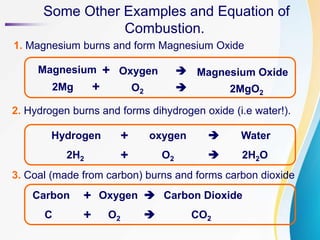
Combustion is the chemical reaction that takes place when a substance burns and reacts with oxygen to produce heat and light energy. Examples of combustible substances that undergo this reaction include paper, wood, coal, and magnesium ribbon. Non-combustible substances like nails and water do not burn. The combustion reaction requires oxygen - when oxygen is removed, a burning candle will go out. Common combustion reactions include magnesium burning to form magnesium oxide, hydrogen burning to form water, and carbon in coal burning to form carbon dioxide.