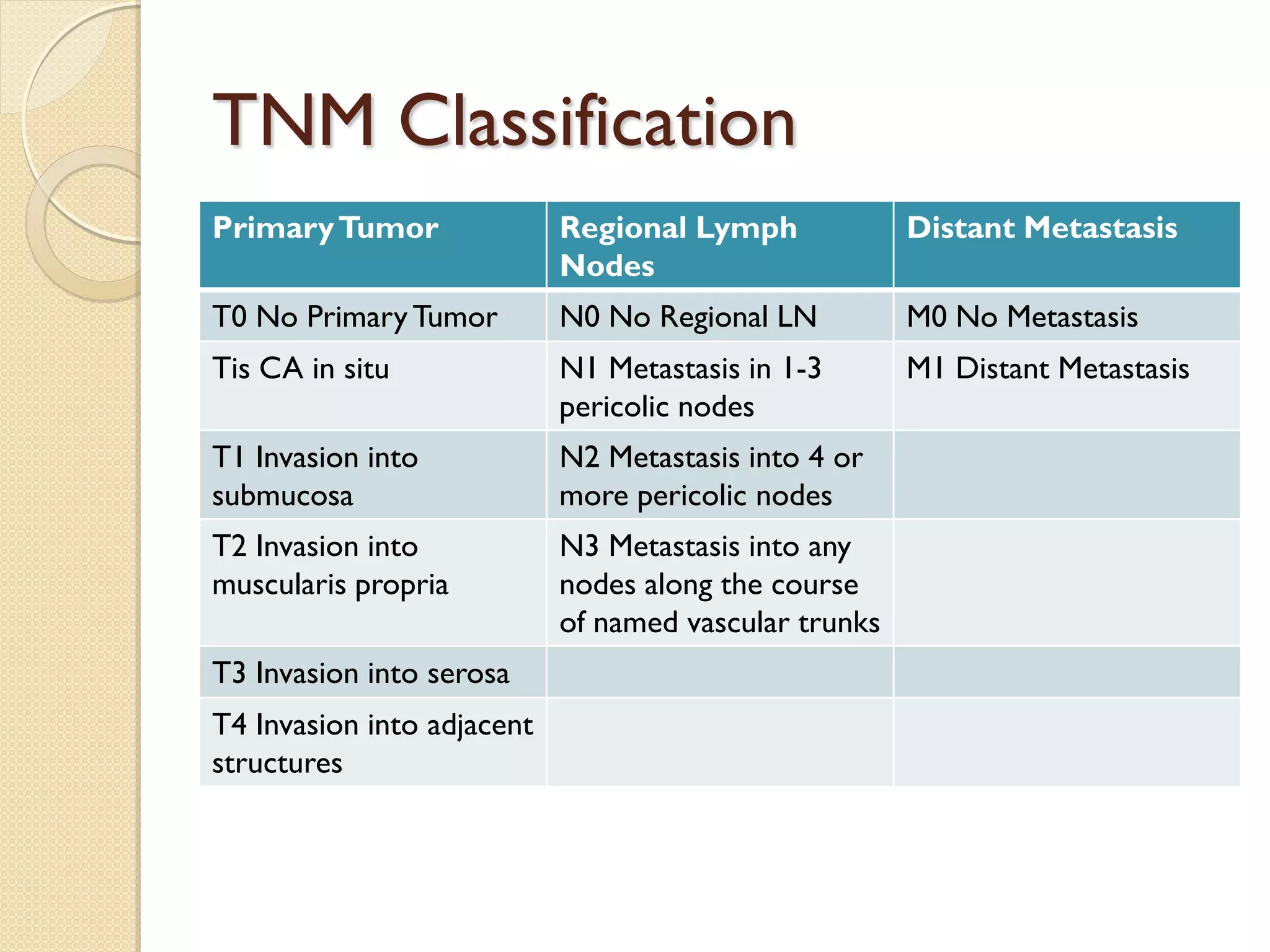
This document discusses colorectal cancer. Some key points:
- Colorectal cancer is the second most common cause of cancer deaths in North America. It affects the colon and rectum.
- Risk factors include family history, age over 50, inflammatory bowel disease, poor diet, smoking, and diabetes. Genetic changes like mutations in APC and DNA repair genes contribute to colorectal cancer development.
- Screening tools include fecal occult blood tests, sigmoidoscopy, colonoscopy, and virtual colonoscopy. Screening guidelines vary but generally recommend annual fecal tests, sigmoidoscopy every 5 years, or colonoscopy every 10 years starting at age 50. Family history of colorectal cancer may