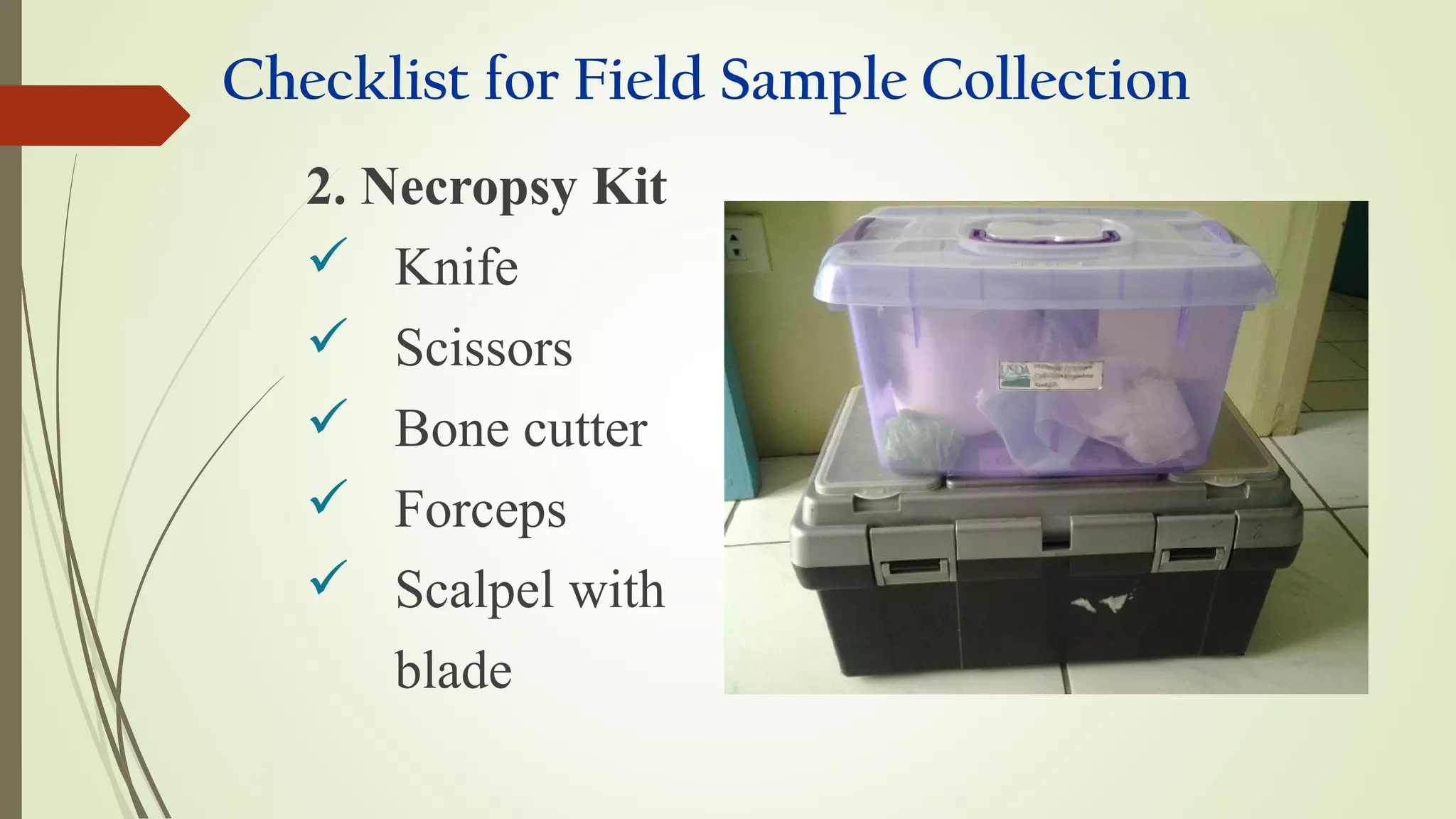
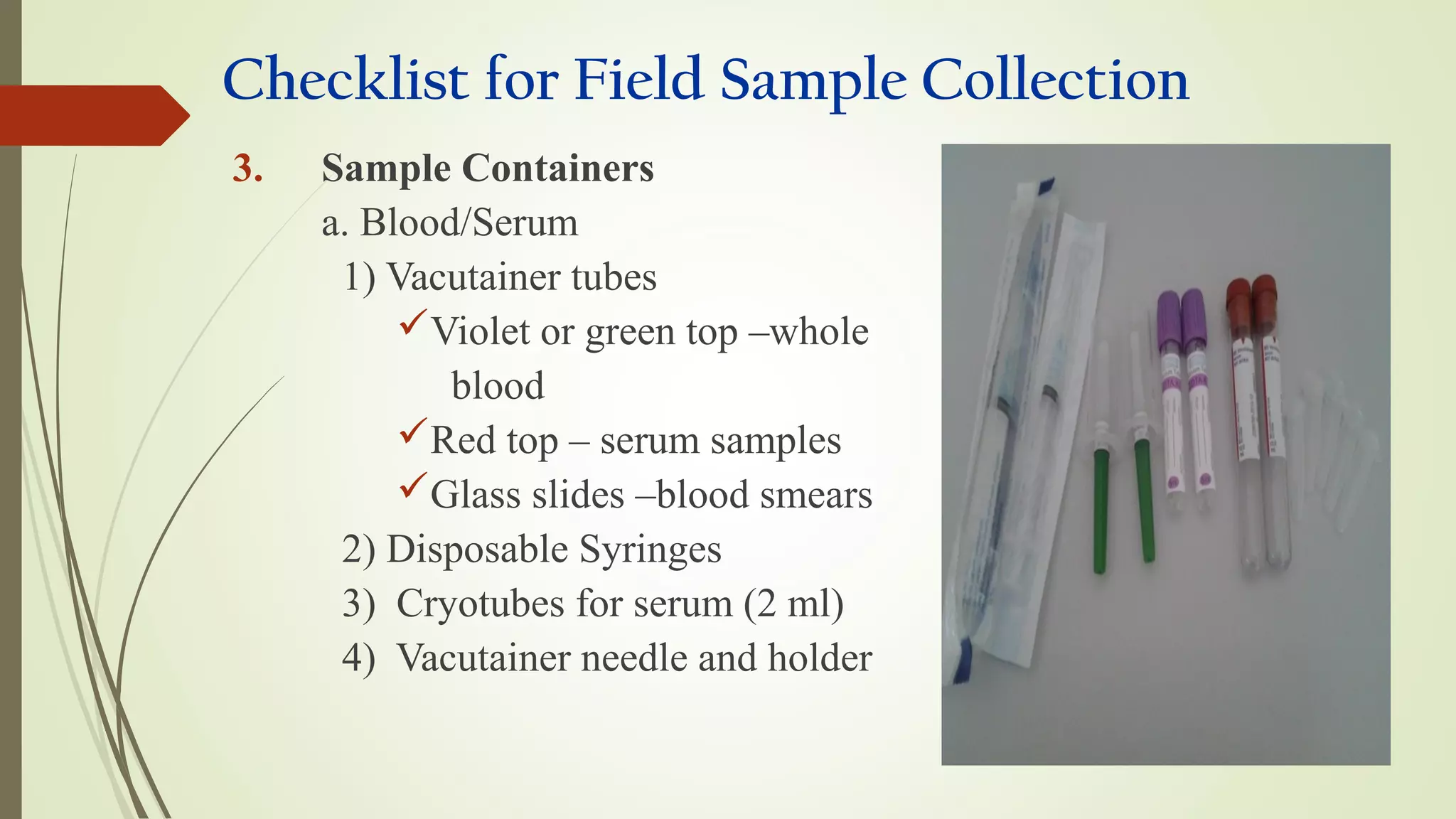
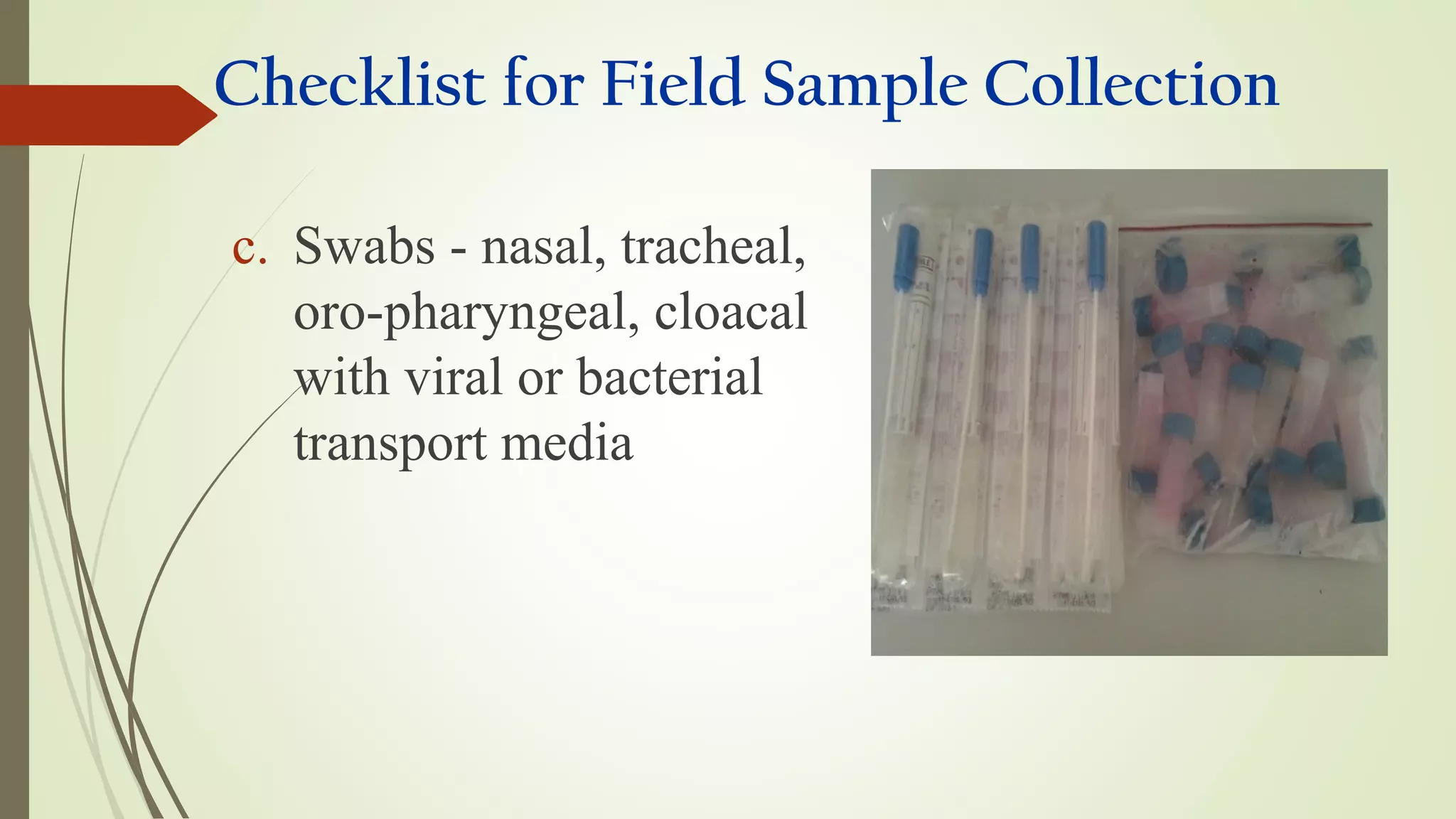
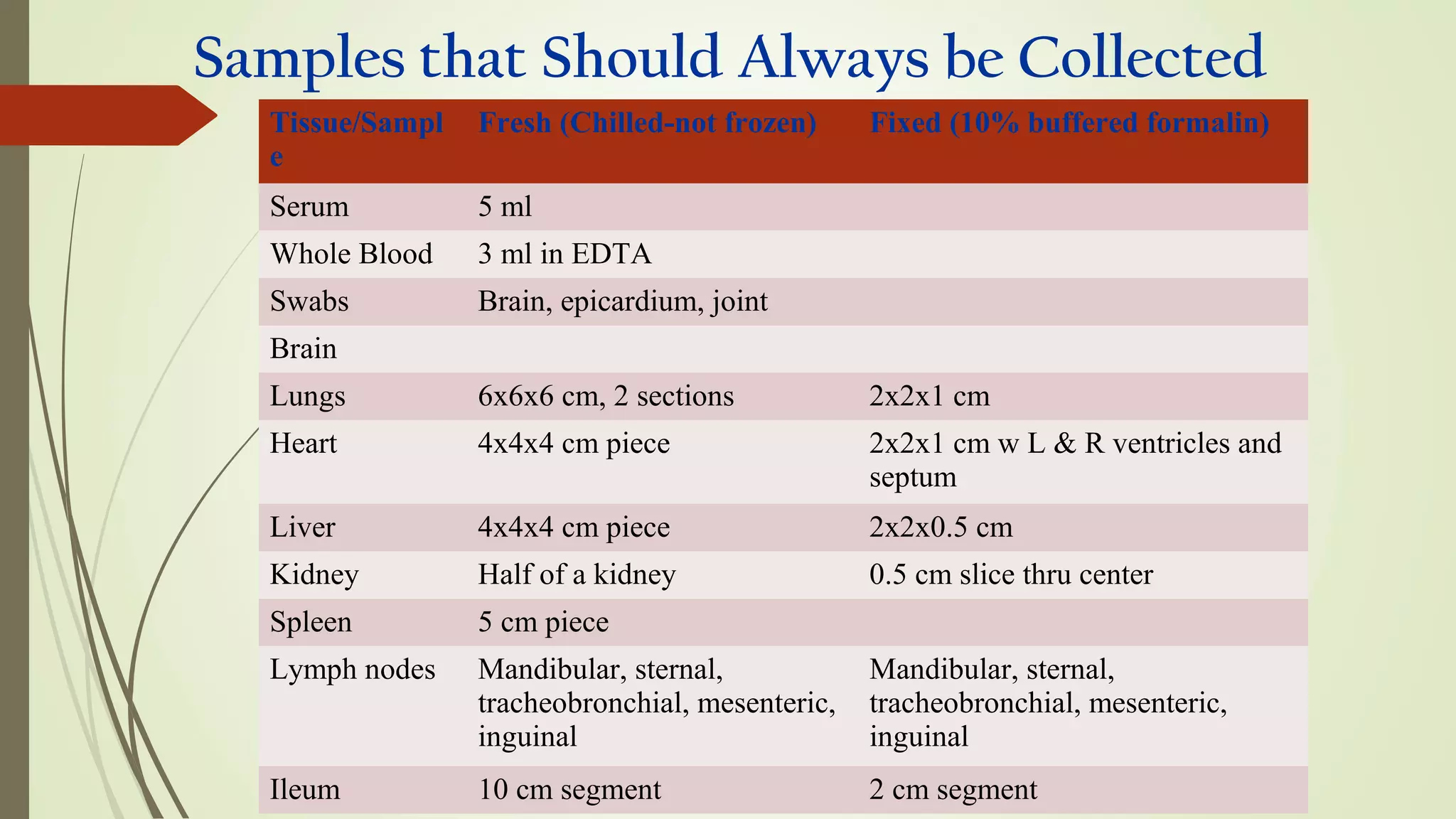
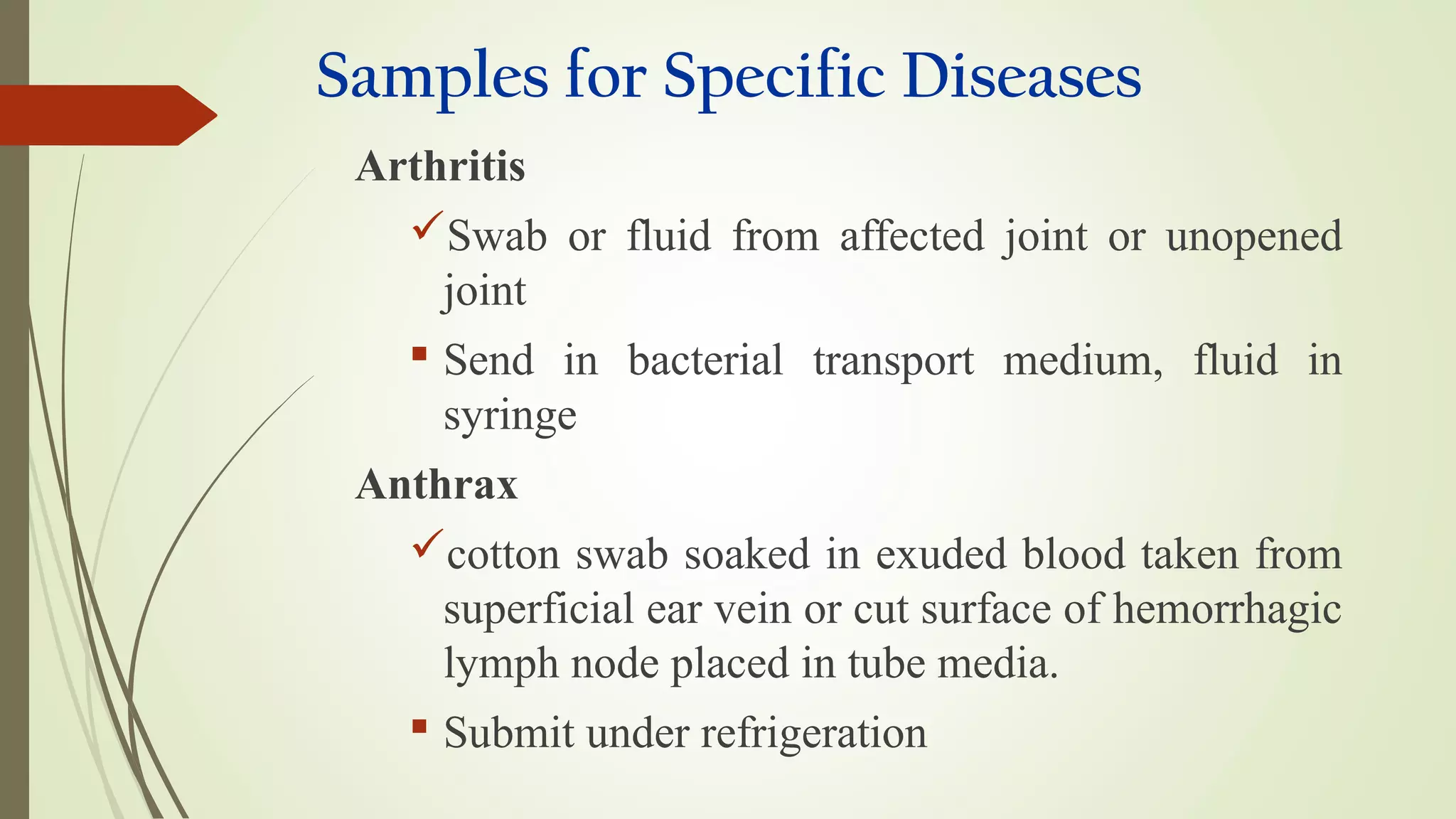
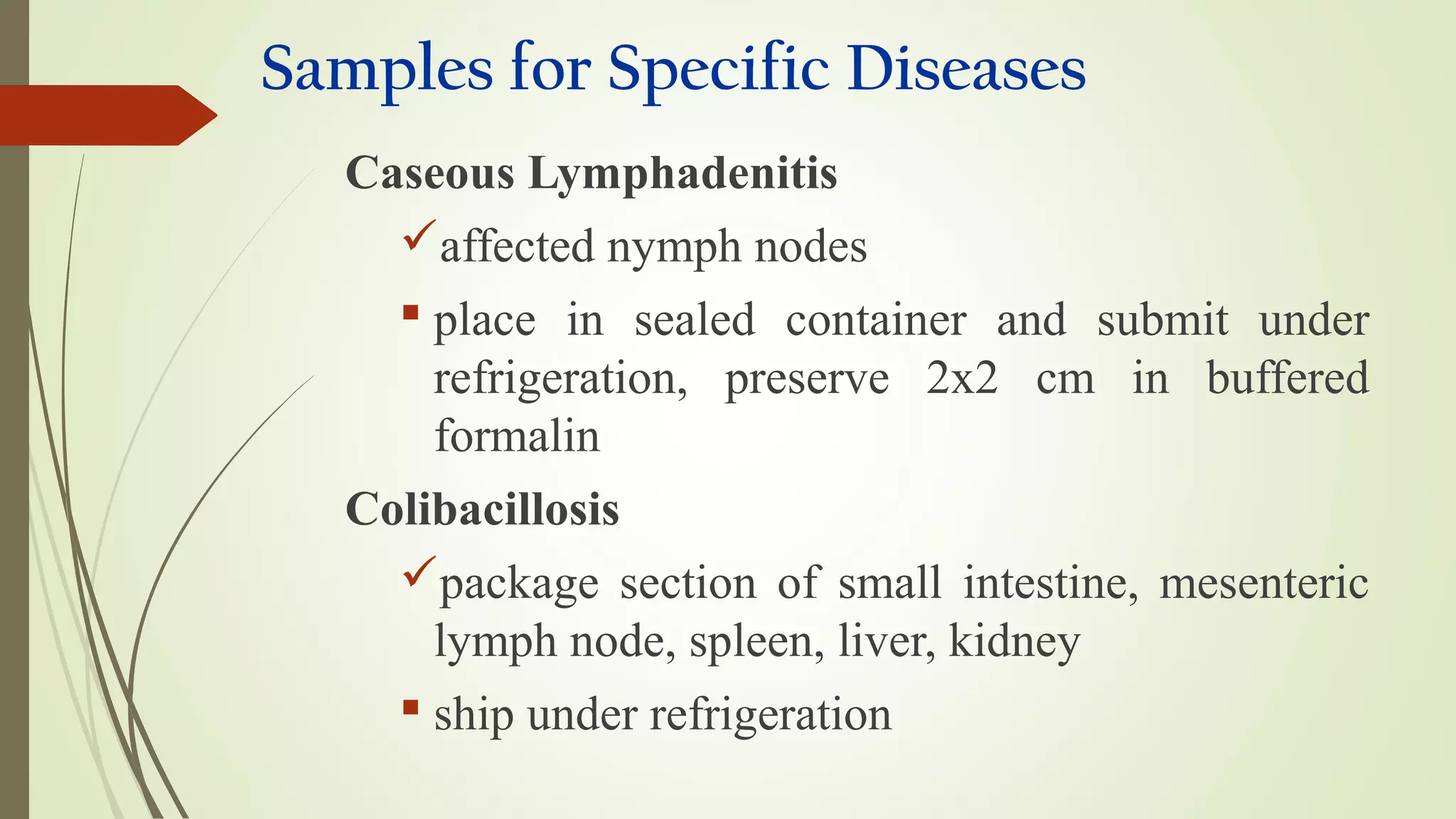
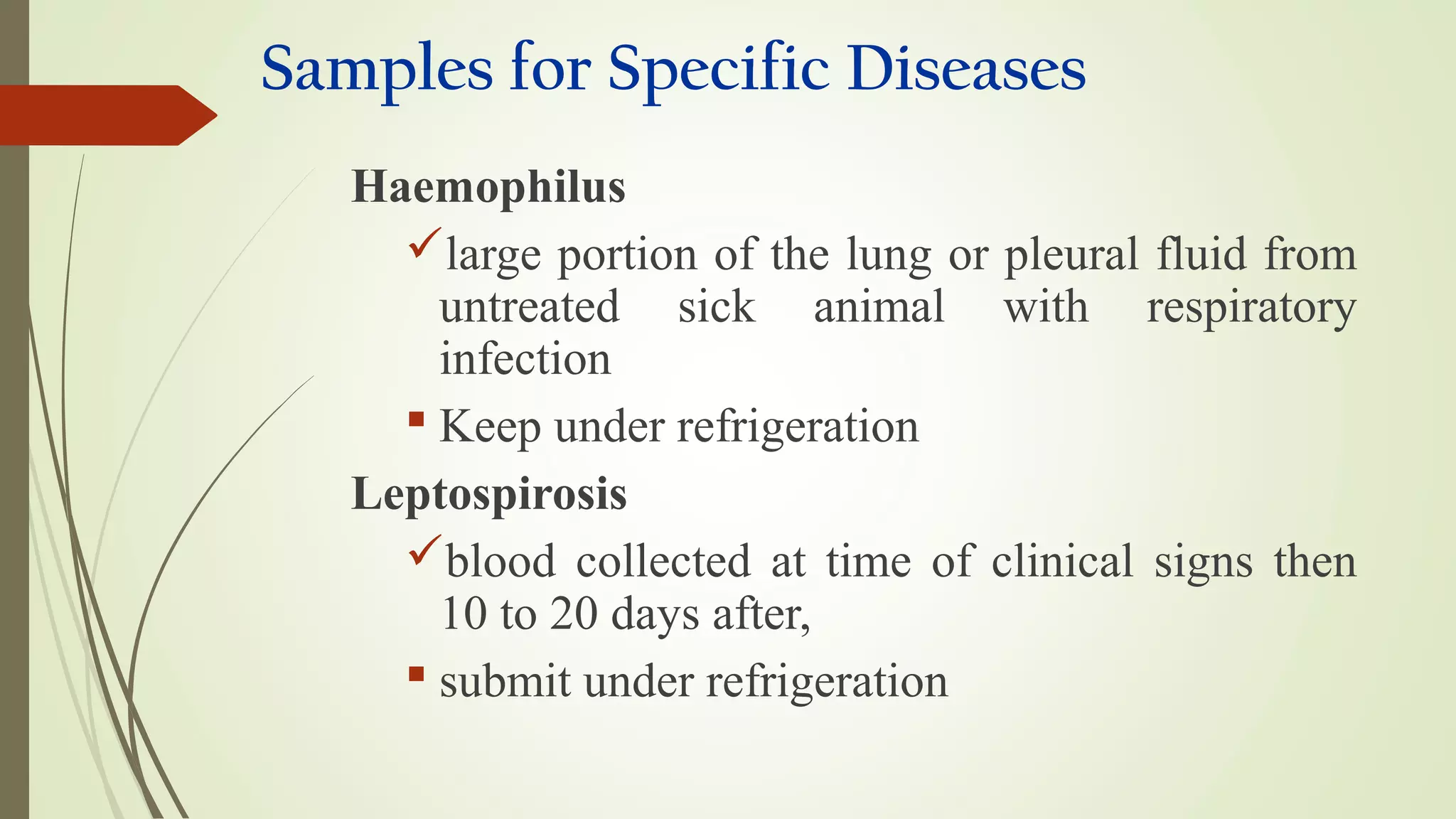
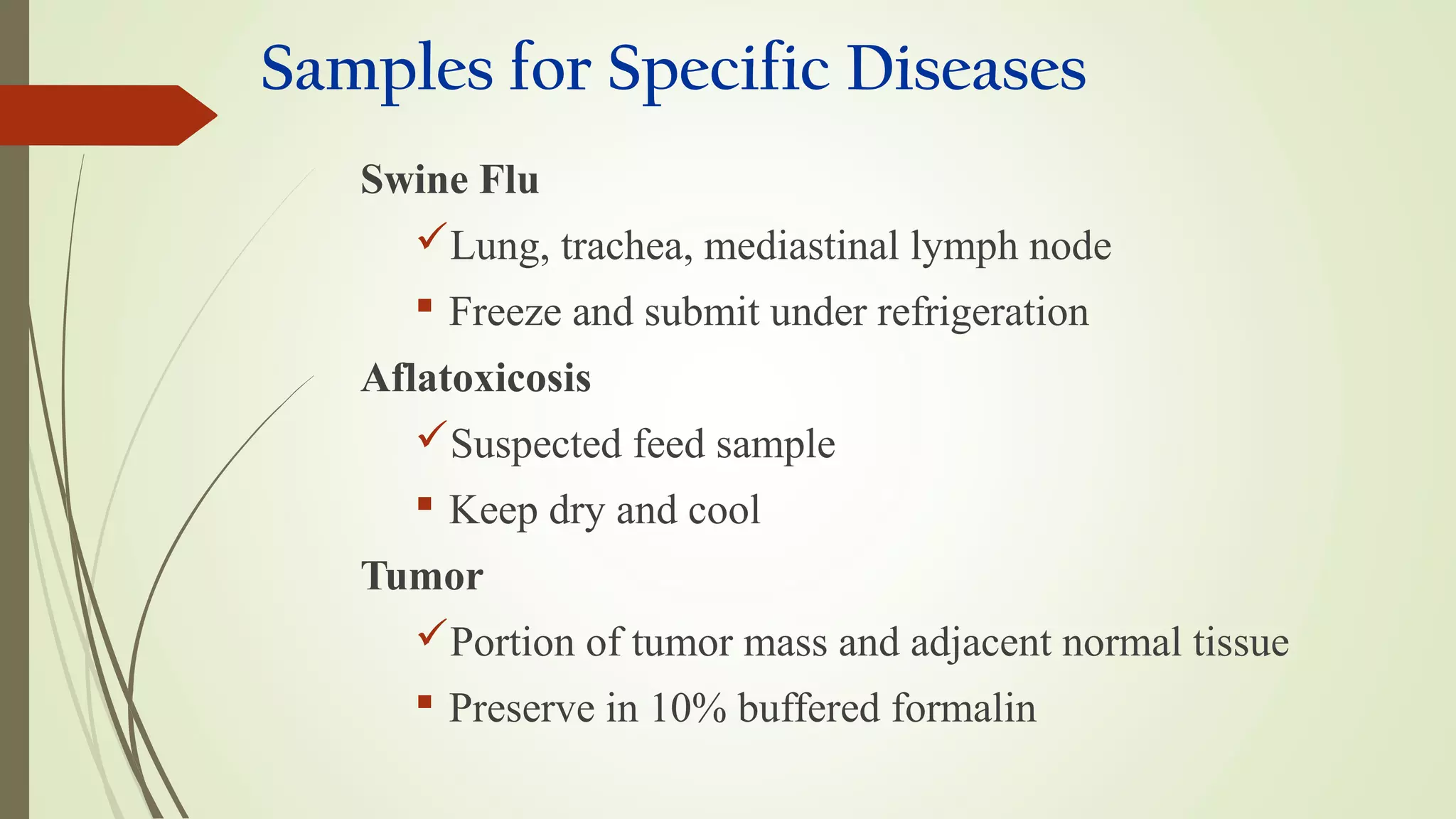
This document provides guidance on collecting, handling, and transporting specimens for laboratory diagnosis of animal diseases. It discusses the purposes of collecting samples, such as for direct examination, isolation of microorganisms, serological and molecular testing. Key steps include using proper protective equipment, collecting sufficient samples before treatment, using sterile containers, maintaining cold chain transport, and providing epidemiological information. A checklist is provided for field sample collection kits and samples that should always be collected from various tissues and body fluids. Proper handling and rapid transport of samples to the laboratory is emphasized.