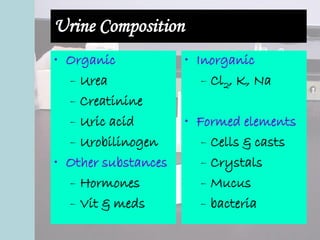
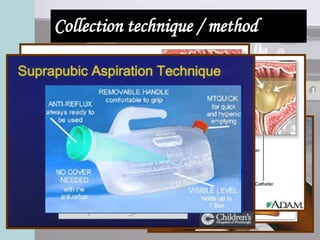
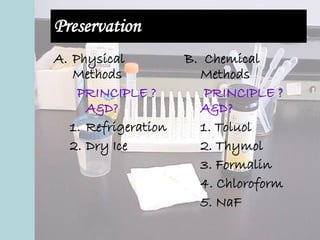
This document provides an overview of urinalysis, including its history, rationale, urine composition, collection techniques, specimen types, and preservation methods. It discusses how urinalysis can provide information about the body's major metabolic functions in a non-invasive way using a readily available specimen. The document outlines factors that can affect urine composition and volume, such as diet, activity level, endocrine functions, and body position. It also describes various urine collection techniques and issues related to specimen handling and preservation.