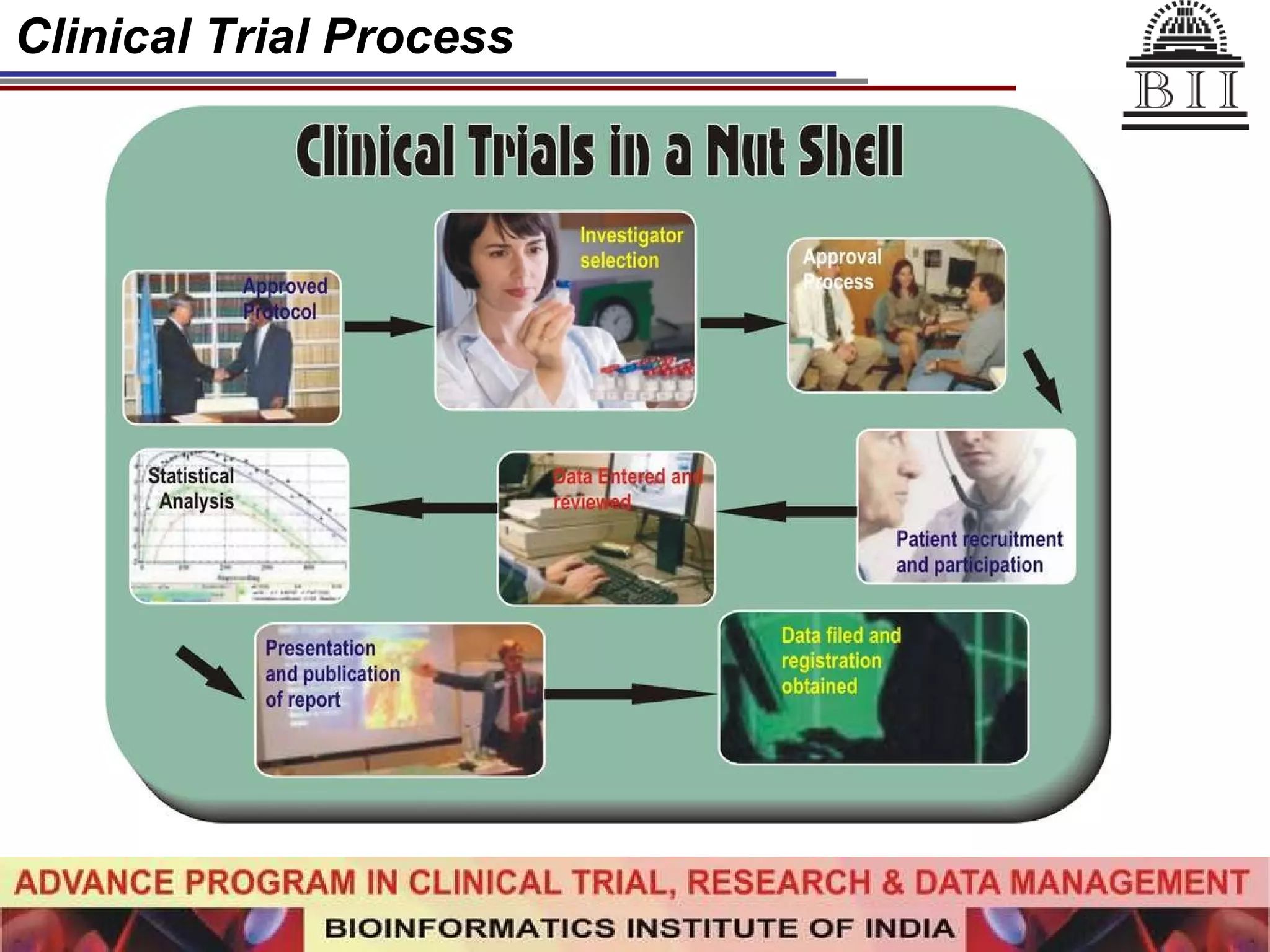
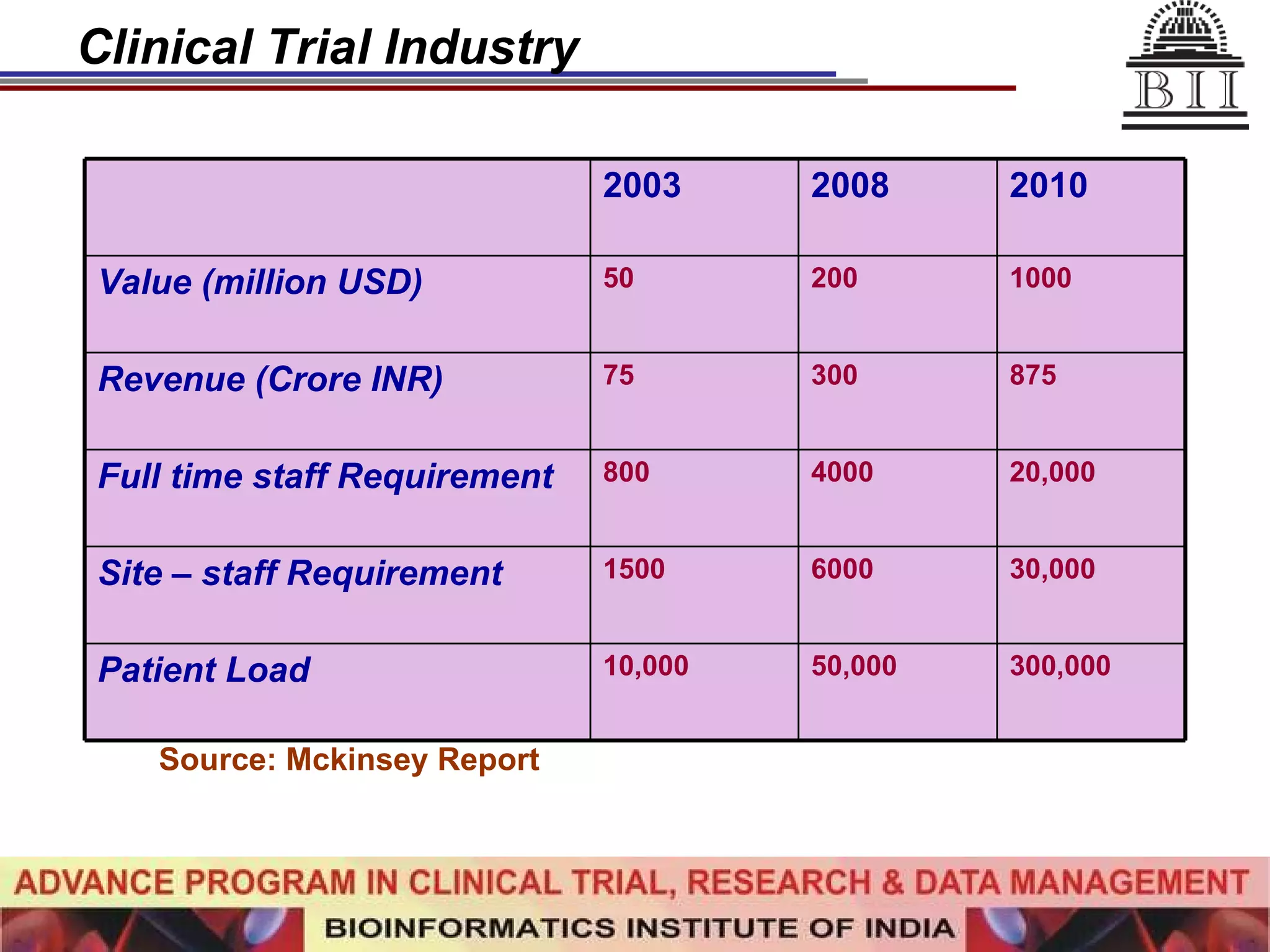
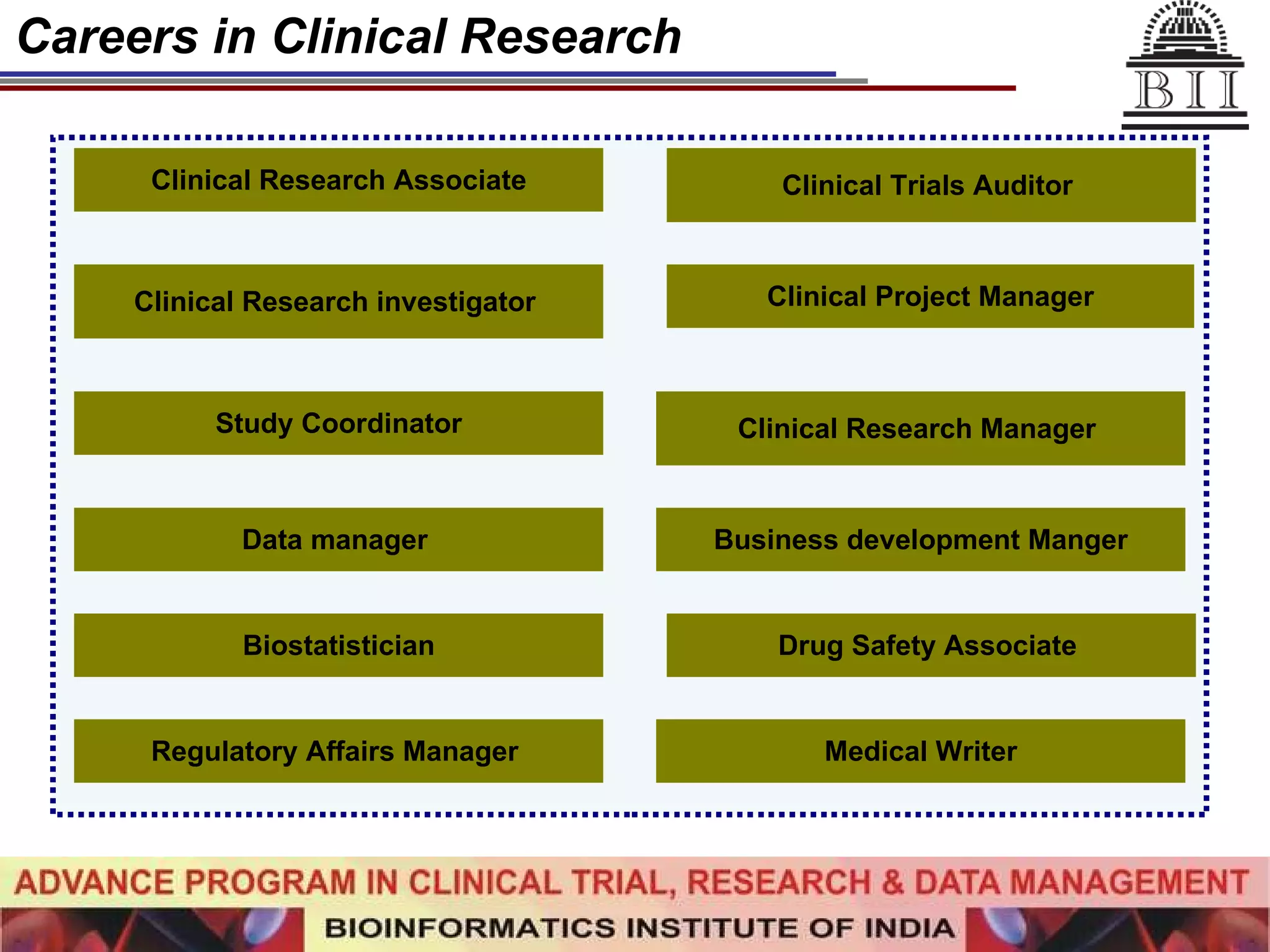
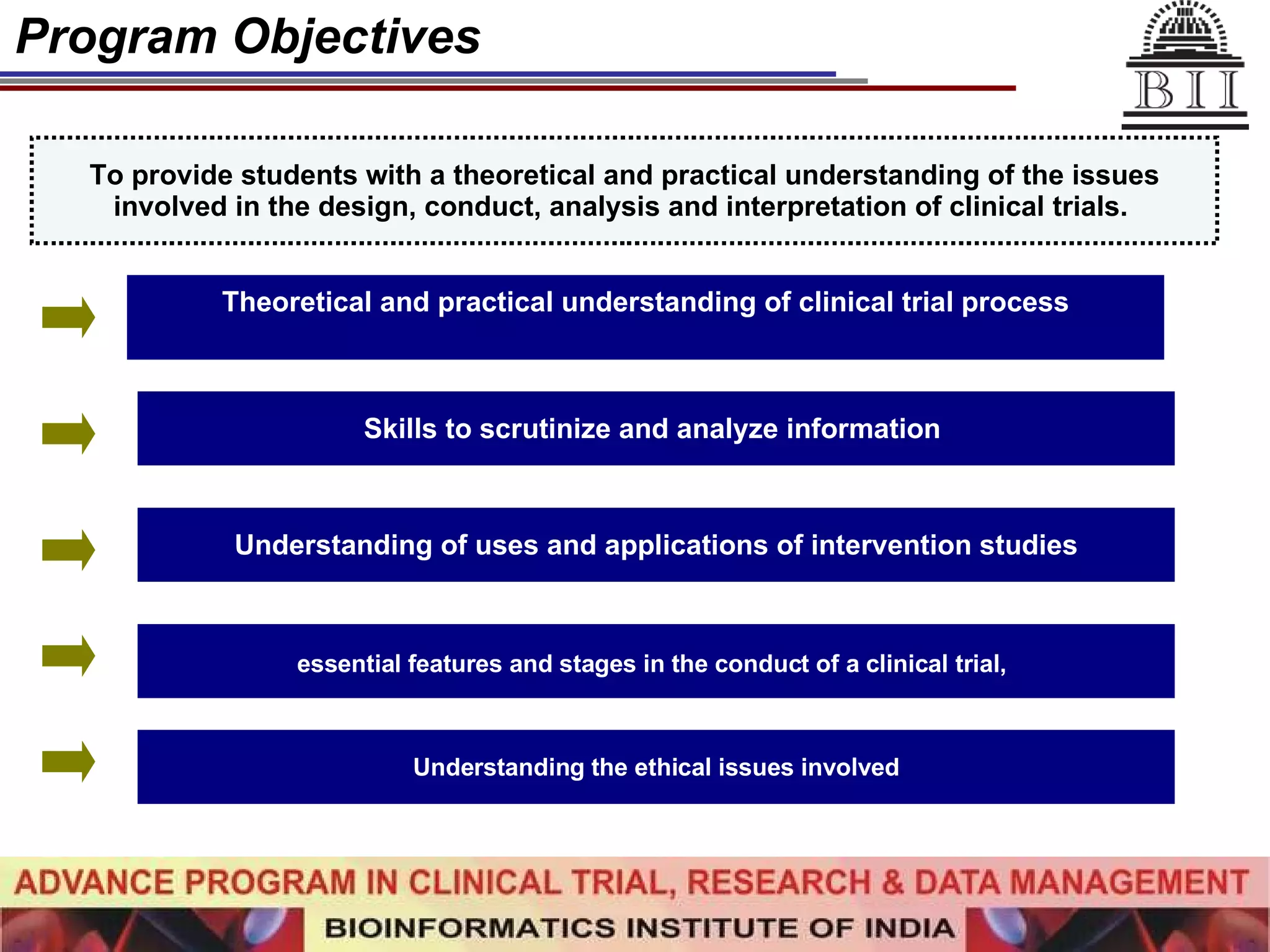
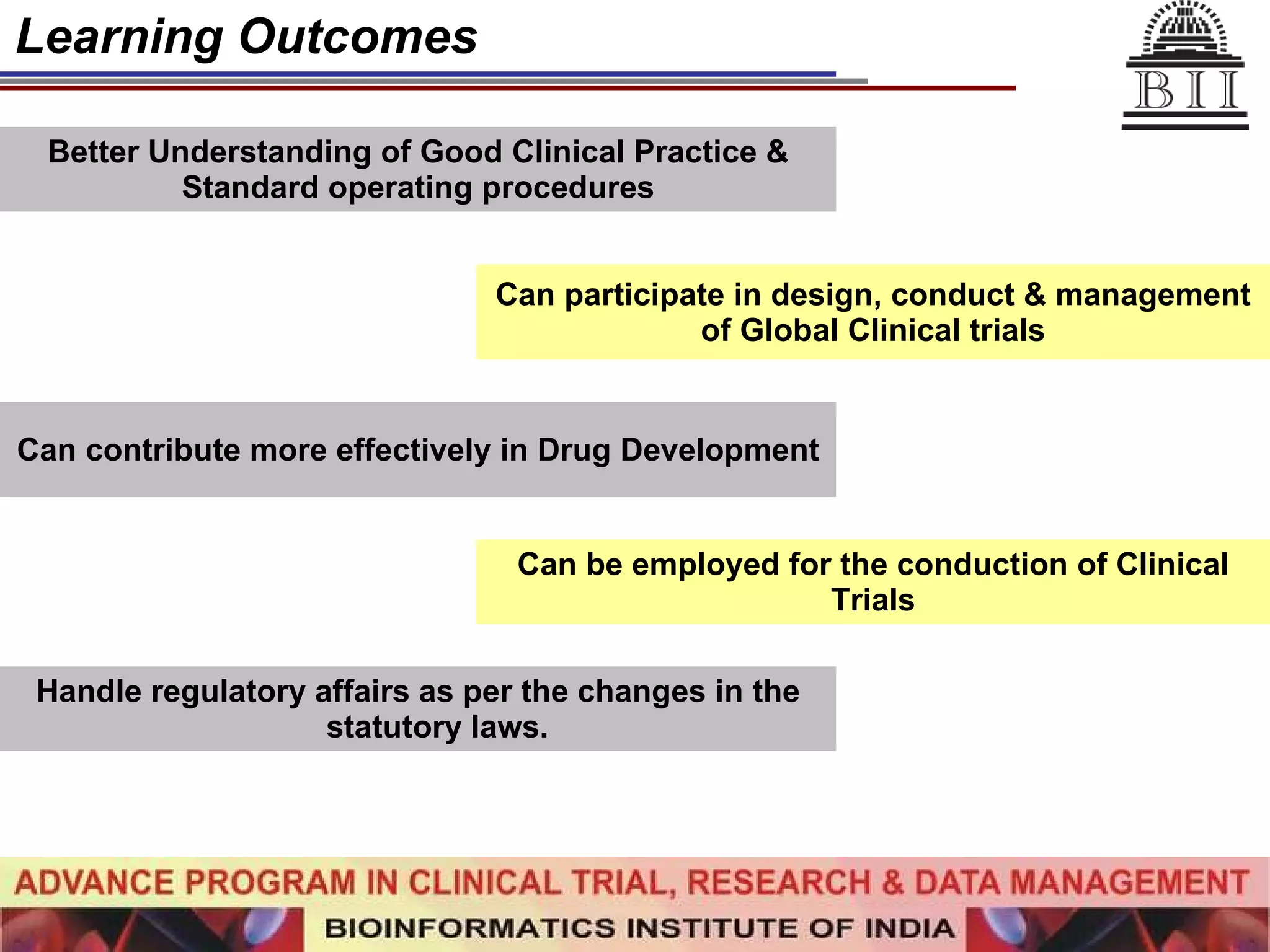
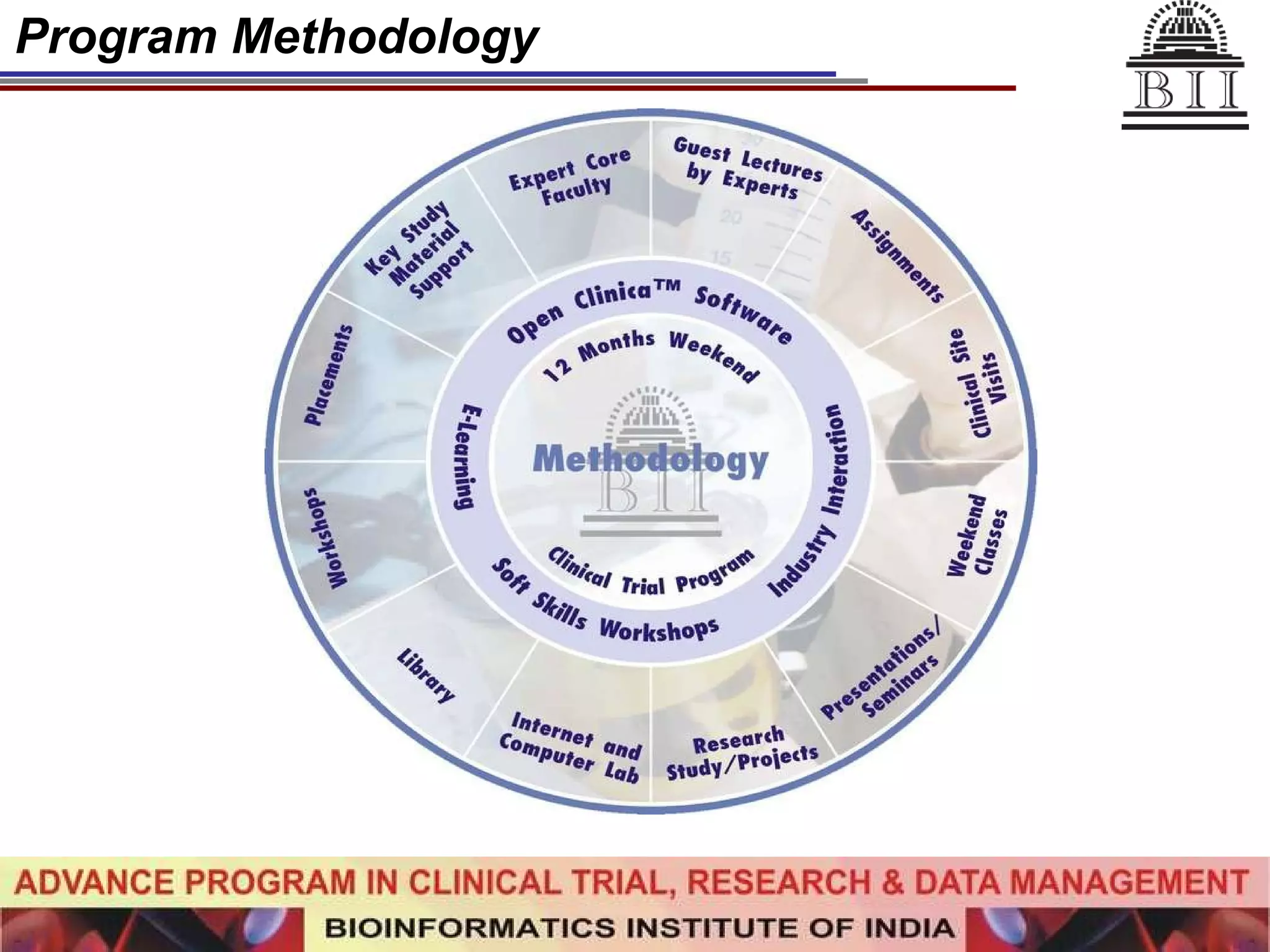
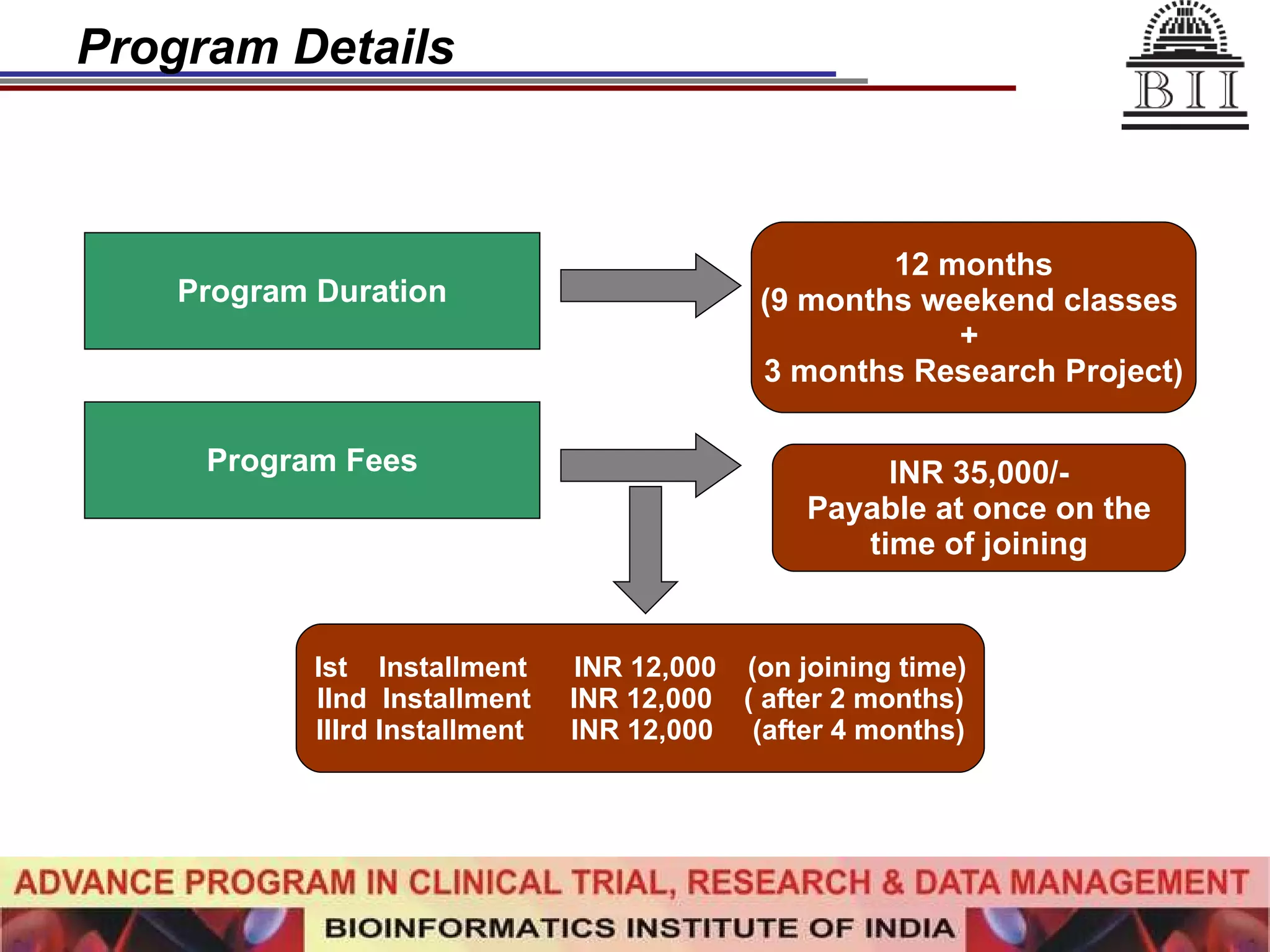
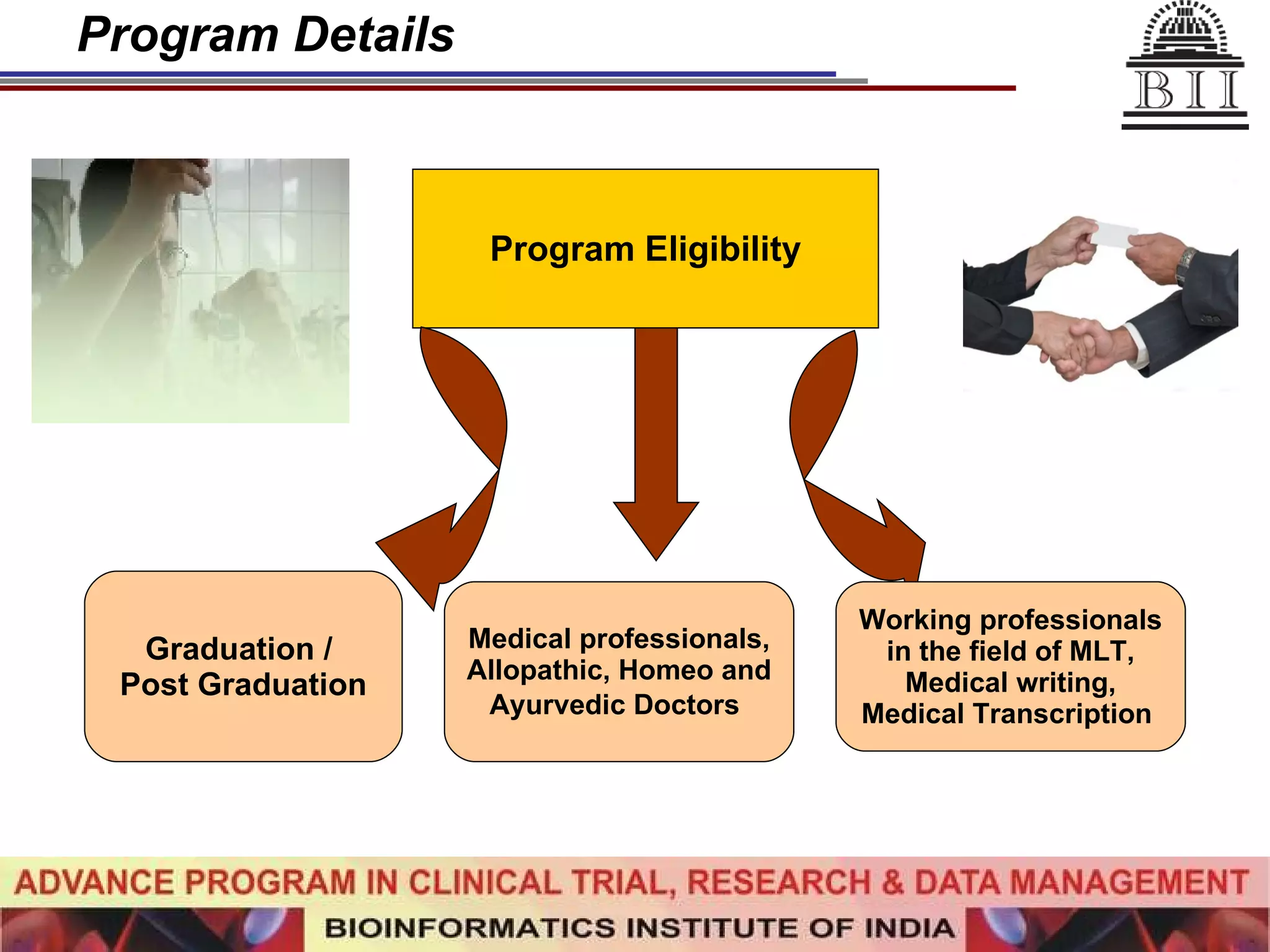
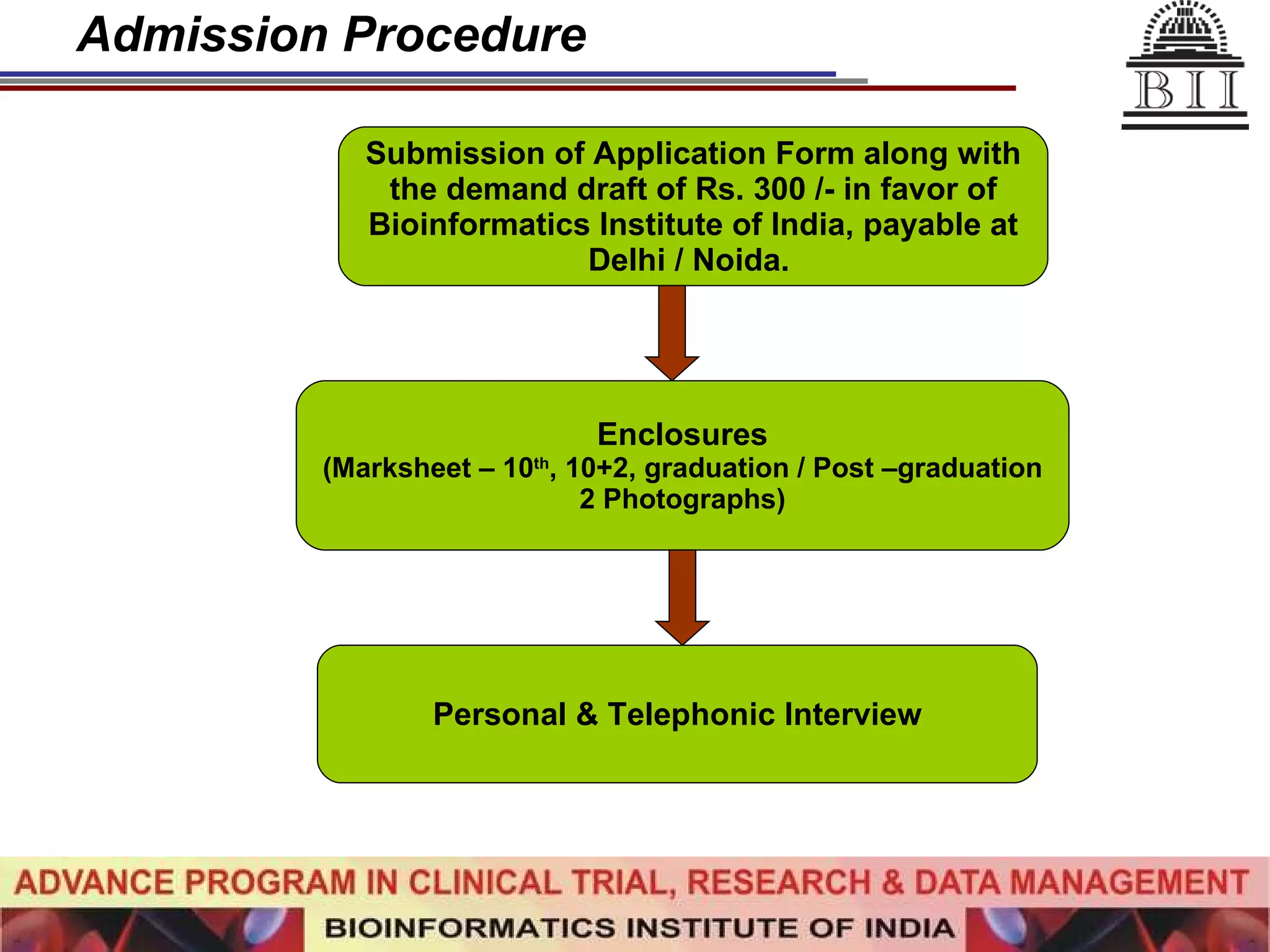
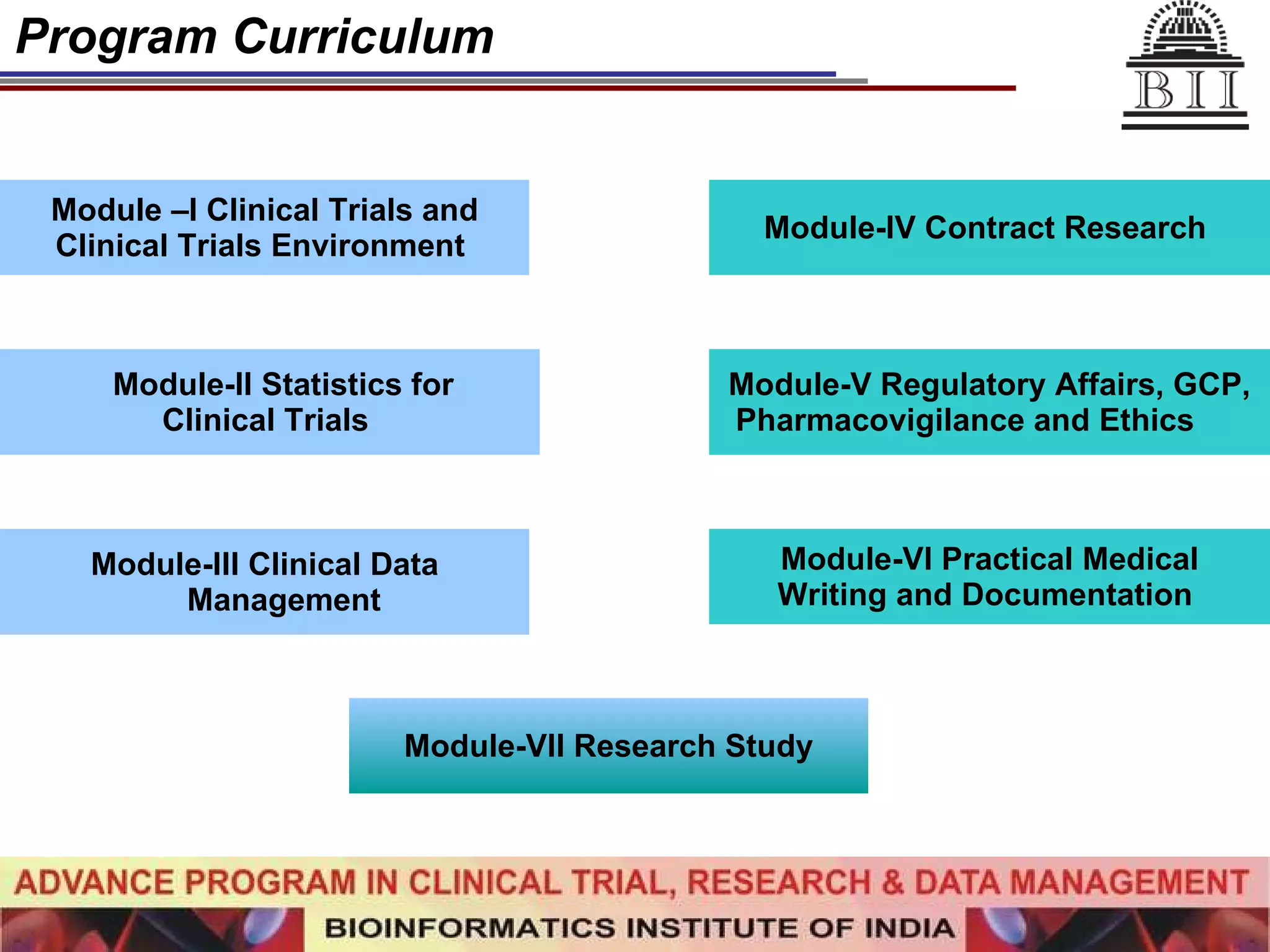
The document advertises an advance program in clinical trial research and data management offered by the Bioinformatics Institute of India. The 12-month weekend program provides theoretical and practical training in clinical trial design, conduct, analysis, and management. It aims to prepare students for careers in the growing clinical research industry in India. The program fee is Rs. 35,000 and includes classroom lectures, guest speakers, research projects, and placement assistance.